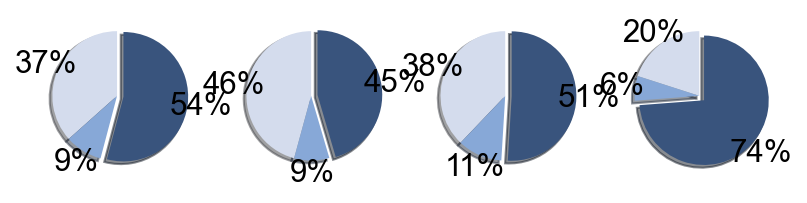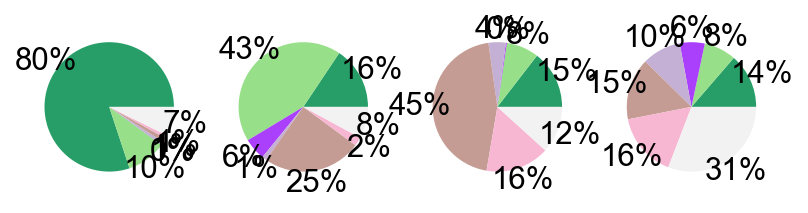(Part II) Analyzing the pan-disease CD8+ T atlas
Contents
(Part II) Analyzing the pan-disease CD8+ T atlas¶
This notebook contains codes for analyzing the pan-disease CD8+ T atlas established from scAtlasVAE.
Please check the package version at https://github.com/WanluLiuLab/scAtlasVAE/blob/master/environment.yml for reproducing the results.
For more information about the scAtlasVAE model, please see https://scatlasvae.readthedocs.io/en/latest/.
For retrieving datasets, please see https://zenodo.org/records/10472914.
SetupEnvironment¶
cd /Volumes/rsch/GEXnb_rev/
/Volumes/rsch/GEXnb_rev
pwd
'/Volumes/rsch/GEXnb_rev'
import time
import xlwt
import gseapy
import numpy as np
import pandas as pd
import matplotlib as mpl
import scanpy as sc
import scirpy as ir
import scvelo as scv
import networkx as nx
import seaborn as sns
import matplotlib.pyplot as plt
from collections import Counter
import scipy
from scipy import stats
from scipy.stats.contingency import odds_ratio
from scipy.integrate import odeint
from matplotlib_venn import venn2, venn3
from scanpy.plotting._anndata import _prepare_dataframe
setPltLinewidth(0)
plt.rcParams.update(plt.rcParamsDefault)
plt.rcParams['figure.dpi'] = 150
plt.rcParams['savefig.dpi'] = 150
plt.rcParams['font.size'] = 15
plt.rcParams['axes.linewidth'] = 0
plt.rcParams['font.family'] = "Arial"
mpl.rcParams['pdf.fonttype'] = 42
mpl.rcParams['ps.fonttype'] = 42
## color_palette
subtype_color = {
'Tn':'#CEBF8F',
'Tcm':'#ffbb78',
'Early Tcm/Tem':'#ff7f0e',
'GZMK+ Tem':'#d62728',
'GNLY+ Temra':'#8c564b',
'CMC1+ Temra':'#e377c2',
'ZNF683+ Teff':'#6f3e7c',
'MAIT':'#17becf',
'ILTCK':'#aec7e8',
'ITGAE+ Trm':'#279e68',
'CREM+ Trm':'#aa40fc',
'ITGB2+ Trm':'#5ce041',
'Tpex':'#ff9896',
'GZMK+ Tex':'#9f80b8',
'ITGAE+ Tex':'#e8c0ba',
'XBP1+ Tex':'#b5bd61',
'MACF1+ T':'#3288c9',
'Cycling T':'#f7b6d2'
}
main_type_color = {
'Tn':'#CEBF8F',
'Tcm/Tem':'#ff7f0e',
'Temra':'#8c564b',
'MAIT':'#17becf',
'ILTCK':'#aec7e8',
'Trm':'#279e68',
'Tex':'#e8c0ba',
'MACF1+T':'#3288c9',
'CyclingT':'#f7b6d2'
}
disease_type_color = {
'Healthy':"#E84C36",
'Infection':"#4CBDD7",
'Inflammation':"#1DA088",
'irAE_inflammation':"#3C5589",
'Solid tumor':"#F49C7F",
'Myeloma':"#8492B5",
'Leukemia':"#91D1C2",
'Fraility':'#1f77b4',
'Organoid':'#aa40fc'
}
meta_tissue_type_color = {
"Circulating":'#ff7f0e',
"Tissue":'#1f77b4',
"TIL":'#279e68',
"Others":'#aa40fc',
}
tissue_type_color = {
'PBMC':'#ffbb78',
'Bone Marrow':'#b5bd61',
'Spleen':'#ff817b',
'Lymph Node':'#e377c2',
'Normal Tissue':'#aec7e8',
'Paratumor Tissue':'#c5b0d5',
'Inflamed Tissue':'#8c564b',
'Infected Tissue':'#ff9896',
'TIL':'#98df8a',
'st_PBMC':'#17becf',
'PBMC/BALF':'#939292',
'Organoid':'#aa40fc',
'Thymus':'#B0BEC5',
}
sorting_color = {
'normal_CD8': '#E6DCD9',
'mem_CD8': '#FFAF00',
'PD1_CD8': '#389663',
'CD39_CD8': '#8256ED',
'st_CD8':'#BBD400',
'ag_specific': '#FF5F5F',
'sorted_CD4': '#009EB2',
'undetermined': '#7F7F7F'
}
## lists
meta_cell_subsets = [
'normal_CD8',
'mem_CD8',
'PD1_CD8',
'CD39_CD8',
'st_CD8',
'ag_specific',
'sorted_CD4',
'undetermined'
]
disease_types = [
'Healthy',
'Infection',
'Inflammation',
'irAE_inflammation',
'Solid tumor',
'Myeloma',
'Leukemia',
'Fraility'
]
disease_types_tex = [
'Healthy',
'Infection',
'Inflammation',
'irAE_inflammation',
'Solid tumor',
]
cell_subtypes = [
'Tn',
'Tcm',
'Early Tcm/Tem',
'GZMK+ Tem',
'GNLY+ Temra',
'CMC1+ Temra',
'ZNF683+ Teff',
'MAIT',
'ILTCK',
'ITGAE+ Trm',
'CREM+ Trm',
'ITGB2+ Trm',
'Tpex',
'GZMK+ Tex',
'ITGAE+ Tex',
'XBP1+ Tex',
'MACF1+ T',
'Cycling T',
]
cell_subtype_aggrs = [
'Tn', 'Tcm/Tem', 'Temra/Teff',
'MAIT', 'ILTCK',
'Trm', 'Tex',
'MACF1+T', 'CyclingT'
]
meta_tissue_type_aggrs = [
"Circulating",
"Tissue",
"TIL",
"Others",
]
meta_tissue_types = [
'PBMC',
'Bone Marrow',
'Spleen',
'Lymph Node',
'Normal Tissue',
'Paratumor Tissue',
'Inflamed Tissue',
'Infected Tissue',
'TIL',
'st_PBMC',
'Organoid',
'Thymus',
]
expansion_levels = [
'No expansion',
'Expansion2-5',
'Expansion6-50',
'Expansion51-100',
'Expansion>100'
]
circ_tissue = [
'Boland_2020', ## UC
'Simone_2021', ## AS
'Kim_2022', ## irAE-Arthritis
'Borcherding_2021', ## ccRCC(PBMC)
'Krishna_2021', ## ccRCC(PBMC)
'Liu_2021', ## NPC(PBMC)
## new datasets
'Shi_2022', #ICC,GBC(PBMC)
'Sun_2022', #GC(PBMC)
'Pai_2023', #NSCLC(LN,not PBMC)
'Rahim_2023', #HNSCC(LN,not PBMC)
'Liu_2022', #BC(LN,not PBMC),
]
LoadData¶
adata_nogex = sc.read_h5ad('source/20240131huARdb_v2_GEX.CD8_nob.hvg4k.vae.processed.h5ad')
adata_gene = sc.read_h5ad('source/20240131huARdb_v2_GEX.CD8.all_genes.h5ad') ## no liver study
clone_subtype = pd.read_csv('source/20240131clone_subtype.csv', index_col=0)
## Subset adata
## tissue_types_subset
adata_circ = adata_nogex[adata_nogex.obs['meta_tissue_type_aggr'].str.contains('Circulating')]
adata_tissue = adata_nogex[adata_nogex.obs['meta_tissue_type_aggr'].str.contains('Tissue')]
adata_tissue_TIL = adata_nogex[(adata_nogex.obs['meta_tissue_type_aggr'].str.contains('Tissue')) |
(adata_nogex.obs['meta_tissue_type_aggr'] == 'TIL')]
adata_tumor_tissue = adata_nogex[(adata_nogex.obs['disease_type']=='Solid tumor')&
(adata_nogex.obs['meta_tissue_type_aggr']=='Normal Tissue')]
adata_tissue_TIL = adata_tissue_TIL[~adata_tissue_TIL.obs.index.isin(adata_tumor_tissue.obs.index)]
[SKIP]ClonotypeTableProcessing¶
def clone_df_generate(adata):
clonotype_composition = pandas_aggregation_to_wide ( adata.obs.groupby("cc_aa_identity").agg({
"cell_subtype_3": lambda x: dict(Counter(x))
}) )
clone_df = pd.DataFrame(list(clonotype_composition['cell_subtype_3'].to_numpy()),
index=clonotype_composition['cc_aa_identity']).fillna(0)
clone_df = clone_df[cell_subtypes]
clone_df['total'] = clone_df.iloc[:,0:18].sum(axis=1)
clone_df['total_Tcmem'] = clone_df.iloc[:,1:4].sum(axis=1)
clone_df['total_Temra'] = clone_df.iloc[:,4:7].sum(axis=1)
clone_df['total_Trm'] = clone_df.iloc[:,9:12].sum(axis=1)
clone_df['total_Tex'] = clone_df.iloc[:,13:16].sum(axis=1)
## add cell source
meta_tissue_type_composition = pandas_aggregation_to_wide ( adata.obs.groupby("cc_aa_identity").agg({
"meta_tissue_type_aggr": lambda x: dict(Counter(x))
}) )
clone_df = clone_df.join(pd.DataFrame(list(
meta_tissue_type_composition['meta_tissue_type_aggr'].to_numpy()
), index=meta_tissue_type_composition['cc_aa_identity']).fillna(0))
for i in ['individual_id', 'study_name', 'sample_name', 'meta_cell_subset', 'disease', 'disease_type']:
cloneid_to_obs = dict(zip(adata.obs['cc_aa_identity'], adata.obs[i]))
clone_df[i] = list(map(cloneid_to_obs.get, clone_df.index))
## define expansion
clone_df.loc[clone_df['total']>=3, 'expansion']='Expanded'
clone_df.loc[clone_df['total']<3, 'expansion']='NoExpanded'
## define dominant clonotype
## define ambiguous clonotype with equal maximum value
_clone = clone_df.iloc[:,0:17]
clone_df['dominant'] = _clone.idxmax(axis=1)
clone_df['dominant_num'] = _clone.max(axis=1)
clone_df['non_dominant_num'] = clone_df['total']-clone_df['dominant_num']
amb_id = _clone.eq(_clone.max(axis=1), axis=0).sum(axis=1)
clone_df['dominant'] = clone_df['dominant'].mask(amb_id > 1, 'Equality')
return clone_df
ClonotypeDefinition¶
TRAB_DEFINITION_ORIG
['IR_VJ_1_junction_aa',
'IR_VDJ_1_junction_aa',
'IR_VJ_1_v_call',
'IR_VJ_1_j_call',
'IR_VDJ_1_v_call',
'IR_VDJ_1_j_call']
adata_nogex.obs['tcr'] = list(map(lambda x: '-'.join(x),
adata_nogex.obs.loc[:,TRAB_DEFINITION_ORIG + ['individual_id']].to_numpy()
))
adata_nogex.obs['tcr']
AAACCTGAGGTGCTAG-10x_Datasets-10k_BEAM-T_Human_A0201_CMV_Flu_Covid_spikein_5pv2 CAAGGSQGNLIF-CASSIRSSYEQYF-TRAV27-TRAJ42-TRBV1...
AAACCTGAGTGGAGTC-10x_Datasets-10k_BEAM-T_Human_A0201_CMV_Flu_Covid_spikein_5pv2 CARNTGNQFYF-CASSLGTGIGYYGYTF-TRAV24-TRAJ49-TRB...
AAACCTGCACGGCGTT-10x_Datasets-10k_BEAM-T_Human_A0201_CMV_Flu_Covid_spikein_5pv2 CAVSAINEKLTF-CASLYGGGGNEQFF-TRAV8-6-TRAJ48-TRB...
AAACCTGCACTTAACG-10x_Datasets-10k_BEAM-T_Human_A0201_CMV_Flu_Covid_spikein_5pv2 CAGGGSQGNLIF-CASSIRSSYEQYF-TRAV27-TRAJ42-TRBV1...
AAACCTGCAGGCTCAC-10x_Datasets-10k_BEAM-T_Human_A0201_CMV_Flu_Covid_spikein_5pv2 CAADDTNTGNQFYF-CASLYGGGGNEQFF-TRAV41-TRAJ49-TR...
...
TTAGTTCAGCTAAACA-Simone_2021-AS02_SF CAFLDDQGGKLIF-CASSAGAGGADTQYF-TRAV24-TRAJ23-TR...
TTCCCAGCAATGCCAT-Simone_2021-AS02_SF CATHGDSGYALNF-CASSWGQSYEQYF-TRAV17-TRAJ41-TRBV...
TTCGAAGAGCTAACTC-Simone_2021-AS02_SF CAASTAQGGSEKLVF-CASSYWGDQPQHF-TRAV23DV6-TRAJ57...
TTGCCGTCAATGCCAT-Simone_2021-AS02_SF CAVGNTDKLIF-CASTTGEYQPQHF-TRAV39-TRAJ34-TRBV28...
TTGCGTCTCGGATGTT-Simone_2021-AS02_SF CAANSPSSNTGKLIF-CSARDQGSGLIYEQYF-TRAV29DV5-TRA...
Name: tcr, Length: 1151695, dtype: object
cloneid = dict(zip(np.unique(adata_nogex.obs['tcr']), list(range(len(np.unique(adata_nogex.obs['tcr']))))))
adata_nogex.obs['cc_aa_identity'] = list(map(cloneid.get, adata_nogex.obs['tcr']))
len(np.unique(adata_nogex.obs['tcr']))
498679
clone_subtype = clone_df_generate(adata_nogex)
clonesize_to_obs = dict(zip(clone_subtype.index, clone_subtype['total']))
adata_nogex.obs['cc_aa_identity_size'] = list(map(clonesize_to_obs.get, adata_nogex.obs['cc_aa_identity']))
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size']==1,
'expansion_level']='No expansion'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size'].isin(range(2,6)),
'expansion_level']='Expansion2-5'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size'].isin(range(6,51)),
'expansion_level']='Expansion6-50'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size'].isin(range(51,101)),
'expansion_level']='Expansion51-100'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size']>100,
'expansion_level']='Expansion>100'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size']==1, 'expansion_size']='1'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size']==2, 'expansion_size']='2'
adata_nogex.obs.loc[adata_nogex.obs['cc_aa_identity_size']>=3, 'expansion_size']='Larger3'
SharingClonotype¶
def sharing_info(clone_info):
clone_info_expanded = clone_info[(clone_info['total']>=3)]
for i in cell_subtypes:
## larger than 20% of cells
clone_info_expanded.loc[clone_info_expanded[i]>=0.2*clone_info_expanded['total'], i]=i
clone_info_expanded.loc[clone_info_expanded[i]!=i, i]=None
clone_info_expanded['sharing_type'] = clone_info_expanded.loc[:, cell_subtypes].apply(
lambda x: '='.join(x.dropna().astype(str)), axis=1
)
clone_info_expanded['sharing_type'] = clone_info_expanded['sharing_type'].mask(
~clone_info_expanded['sharing_type'].str.contains('='), 'NoSharing')
clone_info['sharing_type'] = clone_info_expanded['sharing_type'] ## transfer
return clone_info
def dominant_sharing_info(clone_info, expanded=3):
clone_info_expanded = clone_info[(clone_info['total']>=expanded)]
clone_info_expanded_number = clone_info_expanded.iloc[:,0:18]
clone_info_expanded_number['Cycling T'] = 0 ## exclude cycling
## for i in cell_subtypes[:-1]:
## ## clone_info_expanded_number.loc[clone_info_expanded_number[i]<3, i]=0
## clone_info_expanded_number.loc[
## clone_info_expanded_number[i]<0.2*clone_info_expanded['total'], i]=0
clone_info_expanded['first'] = clone_info_expanded_number.columns[
clone_info_expanded_number.values.argsort(1)[:, -1]]
clone_info_expanded['second'] = clone_info_expanded_number.columns[
clone_info_expanded_number.values.argsort(1)[:, -2]]
## require the second not equal to the third
zero_id = clone_info_expanded_number.apply(
lambda row: row.nlargest(2).values[-1],axis=1) == 0
clone_info_expanded['first'] = clone_info_expanded['first'].mask(zero_id, 'NoSharing')
clone_info_expanded['second'] = clone_info_expanded['second'].mask(zero_id, 'NoSharing')
clone_info_expanded_number = clone_info_expanded_number[zero_id==False]
qualified_id = clone_info_expanded_number.apply(
lambda row: row.nlargest(2).values[-1],axis=1) == clone_info_expanded_number.apply(
lambda row: row.nlargest(3).values[-1],axis=1)
qualified_id = qualified_id[qualified_id==True]
clone_info_expanded.loc[clone_info_expanded.index.isin(qualified_id.index), 'first'] = 'Equality'
clone_info_expanded.loc[clone_info_expanded.index.isin(qualified_id.index), 'second'] = 'Equality'
clone_info['first'] = clone_info_expanded['first']
clone_info['second'] = clone_info_expanded['second']
return clone_info
clone_subtype = sharing_info(clone_subtype)
clone_subtype = dominant_sharing_info(clone_subtype)
adata_gene = adata_gene[adata_nogex.obs.index,]
adata_gene.obs = adata_nogex.obs.copy()
## adata_nogex = adata_nogex[~adata_nogex.obs['disease'].str.contains('liver')] ## only 17 cells
## adata_gene = adata_gene[~adata_gene.obs['disease'].str.contains('liver')] ## only 17 cells
## clone_subtype = clone_subtype[clone_subtype['disease'].str.contains('liver')] ## only 17 cells
## adata_nogex.write_h5ad('source/20240131huARdb_v2_GEX.CD8_nob.hvg4k.vae.processed.h5ad')
## adata_gene.write_h5ad('source/20240131huARdb_v2_GEX.CD8.all_genes.h5ad')
## clone_subtype.to_csv('source/20240131clone_subtype.csv')
## R2R
## clone_subtype_exp2 = dominant_sharing_info(clone_subtype, expanded=2)
Part1GEX/TCRfeatures¶
Functions¶
## color palette
from colour import Color
from matplotlib.colors import LinearSegmentedColormap
def make_colormap( colors, show_palette = False ):
color_ramp = LinearSegmentedColormap.from_list( 'my_list', [ Color( c1 ).rgb for c1 in colors ] )
if show_palette:
plt.figure( figsize = (15,3))
plt.imshow( [ list(np.arange(0, len( colors ) , 0.1)) ] , interpolation='nearest', origin='lower', cmap= color_ramp )
plt.xticks([])
plt.yticks([])
return color_ramp
StatsFunctions¶
def odds_ratio_df(adata=adata_nogex,
groupby='meta_cell_subset', groups=meta_cell_subsets,
targetby='cell_subtype_3', targets=cell_subtypes,
subsets=None):
tissue_subtype_hm = pd.DataFrame([])
tissue_subtype_hm.index = targets
for i in groups:
condition_subtypes = dict(Counter(adata.obs[
adata.obs[groupby]==i
][targetby]))
tissue_subtype_hm = pd.concat([tissue_subtype_hm, pd.Series(condition_subtypes, name=str(i))],axis=1)
tissue_subtype_hm.fillna(0, inplace=True)
tissue_subtype_hm.astype(int)
tissue_subtype_hm_odds = pd.DataFrame([])
tissue_subtype_hm_odds.index = targets
for jdx, j in enumerate(tissue_subtype_hm.columns):
tissue_subtype_hm_odds[j+'_odds'] = [
(
(int(tissue_subtype_hm.iloc[i,jdx]),
int(tissue_subtype_hm.sum(axis=1)[i]-tissue_subtype_hm.iloc[i,jdx])),
(int(tissue_subtype_hm.sum(axis=0)[j]-tissue_subtype_hm.iloc[i,jdx]),
int(sum(tissue_subtype_hm.sum(axis=1))-tissue_subtype_hm.iloc[i,jdx]))
) for i in range(len(tissue_subtype_hm.sum(axis=1)))]
if subsets is not None:
tissue_subtype_odds = tissue_subtype_hm_odds[subsets]
else:
tissue_subtype_odds = tissue_subtype_hm_odds
tissue_subtype_odds = tissue_subtype_odds.applymap(
lambda x:scipy.stats.contingency.odds_ratio(x).statistic)
return tissue_subtype_odds
def odds_ratiop_df(adata=adata_nogex,
groupby='meta_cell_subset', groups=meta_cell_subsets,
targetby='cell_subtype_3', targets=cell_subtypes,
subsets=None):
tissue_subtype_hm = pd.DataFrame([])
tissue_subtype_hm.index = targets
for i in groups:
condition_subtypes = dict(Counter(adata.obs[
adata.obs[groupby]==i
][targetby]))
tissue_subtype_hm = pd.concat([tissue_subtype_hm, pd.Series(condition_subtypes, name=str(i))],axis=1)
tissue_subtype_hm.fillna(0, inplace=True)
tissue_subtype_hm.astype(int)
tissue_subtype_hm_odds = pd.DataFrame([])
tissue_subtype_hm_odds.index = targets
for jdx, j in enumerate(tissue_subtype_hm.columns):
tissue_subtype_hm_odds[j+'_odds'] = [
(
(int(tissue_subtype_hm.iloc[i,jdx]),
int(tissue_subtype_hm.sum(axis=1)[i]-tissue_subtype_hm.iloc[i,jdx])),
(int(tissue_subtype_hm.sum(axis=0)[j]-tissue_subtype_hm.iloc[i,jdx]),
int(sum(tissue_subtype_hm.sum(axis=1))-tissue_subtype_hm.iloc[i,jdx]))
) for i in range(len(tissue_subtype_hm.sum(axis=1)))]
if subsets is not None:
tissue_subtype_odds = tissue_subtype_hm_odds[subsets]
else:
tissue_subtype_odds = tissue_subtype_hm_odds
tissue_subtype_odds_p = tissue_subtype_odds.applymap(
lambda x:scipy.stats.fisher_exact(x))
return tissue_subtype_odds_p
## pval = fisher_exact([[20,380],[6,594]]) ### 计算P值
## print(pval)
## print(res.confidence_interval(confidence_level=0.95)) ### 计算置信区间
## ### ConfidenceInterval(low=2.0736853058122384, high=13.092432304669513)
## for i in tissue_subtype_hm.index:
## tissue_subtype_hm.loc[i] = [ x/sum(tissue_subtype_hm.loc[i]) for x in tissue_subtype_hm.loc[i] ]
## D50 stats
def clone_D50(adata, xaxis, xaxis_label, ind=True):
## stats
if ind is True:
group = 'individual_id'
threshold = 100
else:
group = 'sample_name'
threshold = 30
ir.tl.alpha_diversity(adata, groupby=group, target_col='cc_aa_identity',
metric='D50', key_added='D50_sample')
qualified_id = [x for x,y in Counter(adata.obs[group]).items() if y>threshold]
adata = adata[adata.obs[group].isin(qualified_id)]
D50_group = adata.obs[[group, 'D50_sample', xaxis_label]].drop_duplicates(subset=group)
D50_group.index = D50_group[group]
D50_group[xaxis_label] = D50_group[xaxis_label].astype('category')
D50_group['D50_sample'] = D50_group['D50_sample'].astype('float')
D50_group = D50_group.replace(0, np.nan).iloc[:, 1:]
return D50_group
## Gini index calculation
def gini(wealths):
cum_wealths = np.cumsum(sorted(np.append(wealths, 0)))
sum_wealths = cum_wealths[-1]
xarray = np.array(range(0, len(cum_wealths))) / np.float64(len(cum_wealths) - 1)
upper = xarray ## 45 angle line
yarray = cum_wealths / sum_wealths
B = np.trapz(yarray, x=xarray) ## calculate AUC
A = 0.5 - B
G = A / (A + B)
return G
def clone_gini(adata, xaxis, xaxis_label, ind=True):
if ind is True:
group = 'individual_id'
threshold = 100
else:
group = 'sample_name'
threshold = 50
qualified_id = [x for x,y in Counter(adata.obs[group]).items() if y>threshold]
adata = adata[adata.obs[group].isin(qualified_id)]
clone_gini_df = pd.DataFrame([])
sample_num = []
for i in xaxis:
clone_group = []
adata_subgroup = adata[adata.obs[xaxis_label]==i]
sample_subgroup = np.unique(adata_subgroup.obs['individual_id'])
sample_num.append(len(sample_subgroup))
for j in sample_subgroup:
subgroup_sample = adata_subgroup.obs[adata_subgroup.obs['individual_id']==j]
clone_sample = dict(Counter(subgroup_sample.cc_aa_identity))
G = gini(list(clone_sample.values()))
clone_group.append(G)
clone_gini_df = pd.concat([clone_gini_df, pd.Series(clone_group, index=sample_subgroup)], axis=0)
conditions = [np.repeat(i,sample_num[idx]) for idx,i in enumerate(xaxis)]
clone_gini_df[xaxis_label] = [y for x in conditions for y in x ]
clone_gini_df[0] = clone_gini_df[0].astype(float)
return clone_gini_df
## circ_tissue cbind df
def cbind_df(adata_circ, adata_tissue, xaxis, xaxis_label, index_type, ind=True):
if index_type == 'D50':
df1 = clone_D50(adata_circ, xaxis=xaxis, xaxis_label=xaxis_label, ind=ind)
df2 = clone_D50(adata_tissue, xaxis=xaxis, xaxis_label=xaxis_label, ind=ind)
else:
df1 = clone_gini(adata_circ, xaxis=xaxis, xaxis_label=xaxis_label, ind=ind)
df2 = clone_gini(adata_tissue, xaxis=xaxis, xaxis_label=xaxis_label, ind=ind)
df1['origin'] = 'Circulating'
df2['origin'] = 'Tissue'
multi_box = pd.concat([df1, df2], axis=0)
multi_box = multi_box.rename(columns={multi_box.columns[0]: "value", multi_box.columns[1]: "variable"})
multi_box["variable"] = multi_box["variable"].astype('category')
multi_box["variable"] = multi_box["variable"].cat.reorder_categories(xaxis)
return multi_box
PlotsFunctions¶
## general bar function
def general_bar(data, target_col, ylim0, ylim1, file_name):
data = data.sort_values(by=target_col, ascending=False)
width = 0.5
fig,ax = plt.subplots(figsize=(10,8))
ax.set_ylabel(target_col)
ax.set_ylim(ylim0, ylim1)
ax.bar(range(len(data)),
[x-ylim0 for x in data[target_col]], bottom=ylim0,
width=width, align='center', edgecolor=None, color=data['colors'])
ax.set_xticks(range(len(data)))
ax.set_xticklabels(data.index, rotation=45, horizontalalignment='right')
plt.show()
fig.savefig(file_name)
## stacked_bar function
def stacked_bar(adata, percentage, xitems, xitem_str,
yitems, yitem_str, *, yitem_color,
file_name:str):
## stats
stats = []
for j in yitems:
cell_num = [len(adata.obs[(adata.obs[yitem_str]==j) &
(adata.obs[xitem_str]==i)])
for i in xitems]
stats.append(cell_num)
stats = np.array(stats)
## plots
x = range(len(xitems))
width = 0.35
fig,ax = plt.subplots(figsize=(10,8))
bottom_y = np.zeros(len(xitems))
sums = np.sum(stats, axis=0)
for idx,i in enumerate(stats):
if percentage == True:
y = i / sums ## bar height (percentage)
else:
y=i
ax.bar(x, y, width, bottom=bottom_y, edgecolor=None,
label=yitems[idx], color=list(yitem_color.values())[idx])
## bottom position
bottom_y = y + bottom_y
ax.spines['right'].set_visible(False)
ax.spines['top'].set_visible(False)
plt.xticks(x, xitems)
plt.legend(bbox_to_anchor=(1.3, 0.5), loc='center right')
fig.autofmt_xdate(rotation=45)
fig.savefig(file_name)
plt.show()
## pie_plot function
def pie_plot(adata, items, item_str, item_color, file_name):
## SupFigure1BR
## stats
requested_percent = [len(adata.obs[(adata.obs[item_str]==i)]) for i in items]
requested_percent = np.array(requested_percent)
## plots
fig, ax = plt.subplots(figsize=(10,8))
ax.pie(requested_percent, radius=1,
colors=item_color.values(),
autopct='%1.0f%%', pctdistance=1.2,
wedgeprops=dict(width=0.3, edgecolor='w'))
ax.legend(items,
title=item_str,
loc="center left",
fontsize="small",
bbox_to_anchor=(1,0,0.5,1))
fig.savefig(file_name)
plt.show()
## multi boxplot
def multi_boxplot(multi_box, file_name,
xaxis='variable', yaxis='value', hueaxis='origin',
palette_list=disease_type_color.values()):
fig,ax = plt.subplots(figsize=(10,8))
sns.boxplot(x=xaxis, y=yaxis, hue=hueaxis, data=multi_box,
palette=palette_list,
linewidth=0.5, boxprops={"zorder":2},
showfliers=False, ax=ax)
num = len(multi_box[hueaxis].unique())
## Extract x and y coordinates of the dots
sns.stripplot(x=xaxis, y=yaxis, hue=hueaxis,
palette=palette_list, jitter=True,
size=5,
linewidth=1, edgecolor='black',
dodge=0.5 / num * (num - 1),
data=multi_box, ax=ax)
ax.spines['right'].set_visible(False)
ax.spines['top'].set_visible(False)
plt.legend(bbox_to_anchor=(1.3, 0.5), loc='center right')
fig.autofmt_xdate(rotation=45)
fig.savefig(file_name)
plt.show()
MainFigure2¶
## Leiden
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='leiden_n_neighbors_40_reoslution_1.2', size=0.5, ax=ax)

CellSubtypes¶
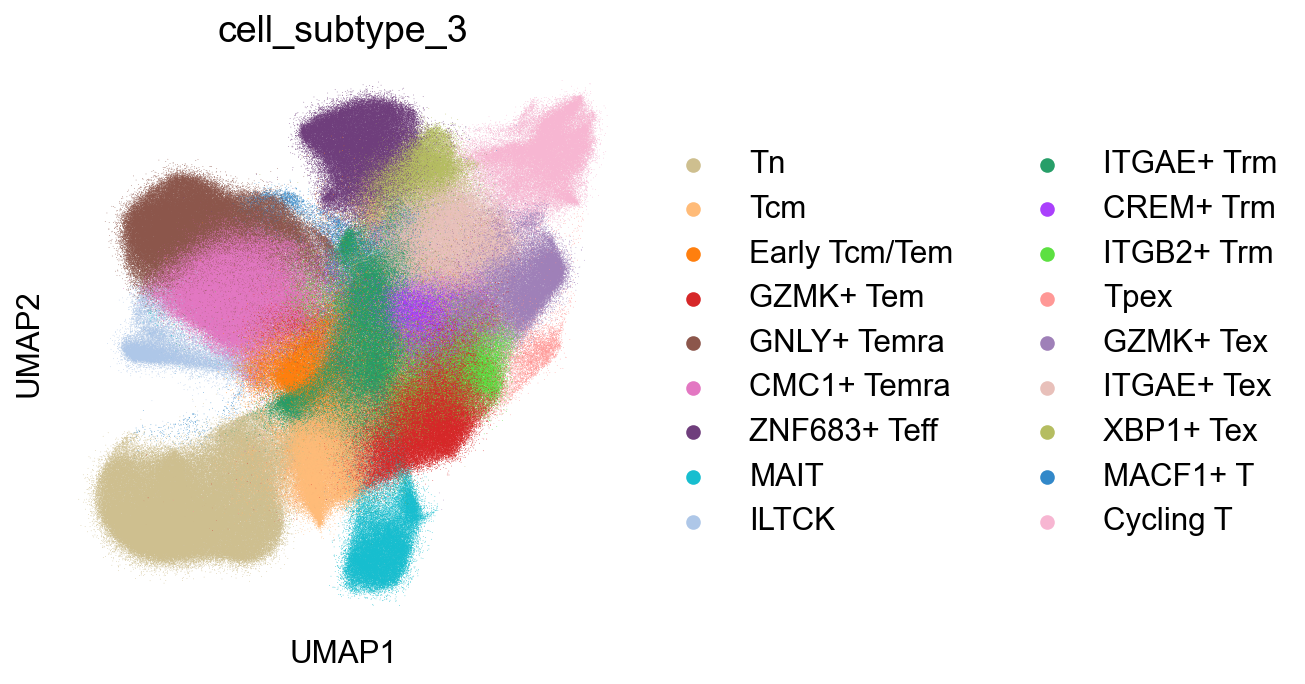
## Cell subtype UMAP, Figure1B
setPltLinewidth(0)
plt.rcParams.update(plt.rcParamsDefault)
plt.rcParams['figure.dpi'] = 150
plt.rcParams['savefig.dpi'] = 150
plt.rcParams['font.size'] = 15
plt.rcParams['axes.linewidth'] = 0
plt.rcParams['font.family'] = "Arial"
mpl.rcParams['pdf.fonttype'] = 42
mpl.rcParams['ps.fonttype'] = 42
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='cell_subtype_3', size=0.5,
ax=ax, palette=subtype_color)
fig.savefig('figures/Figure2A_cell_subtype.png')
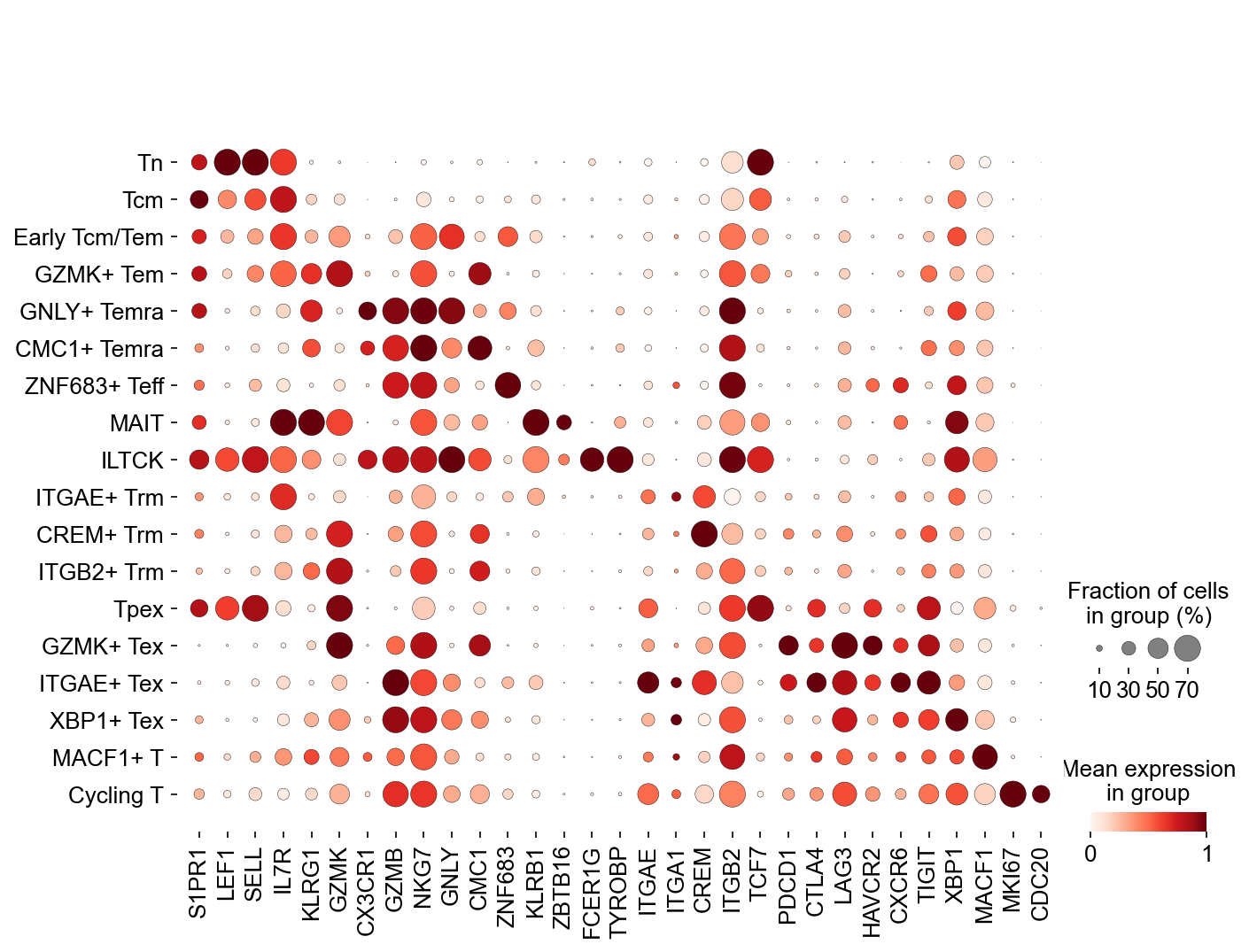
## Cell subtype marker, Figure1C
## Cell subtype marker, Figure1C
fig,ax = plt.subplots(figsize=(10,8))
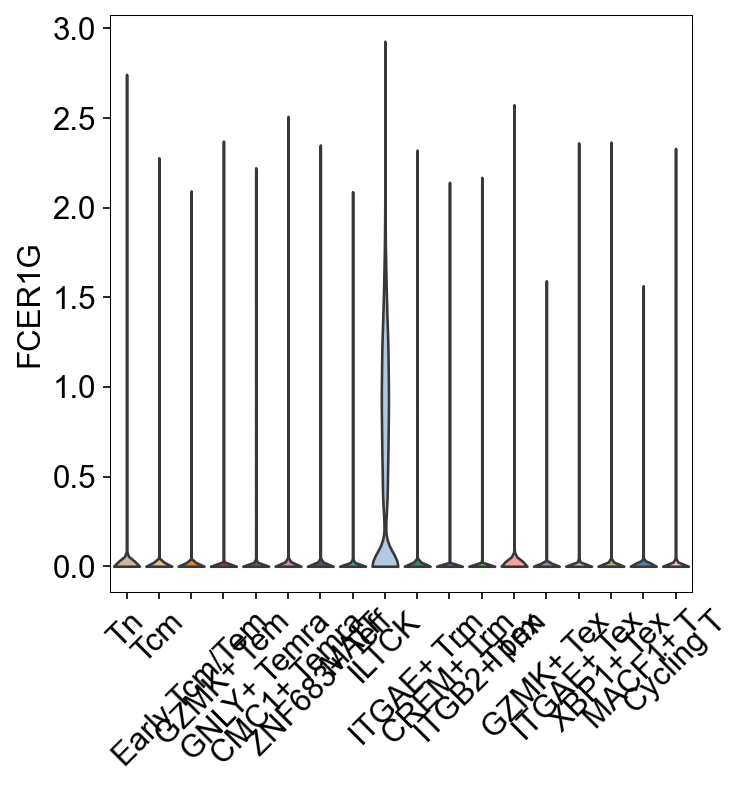
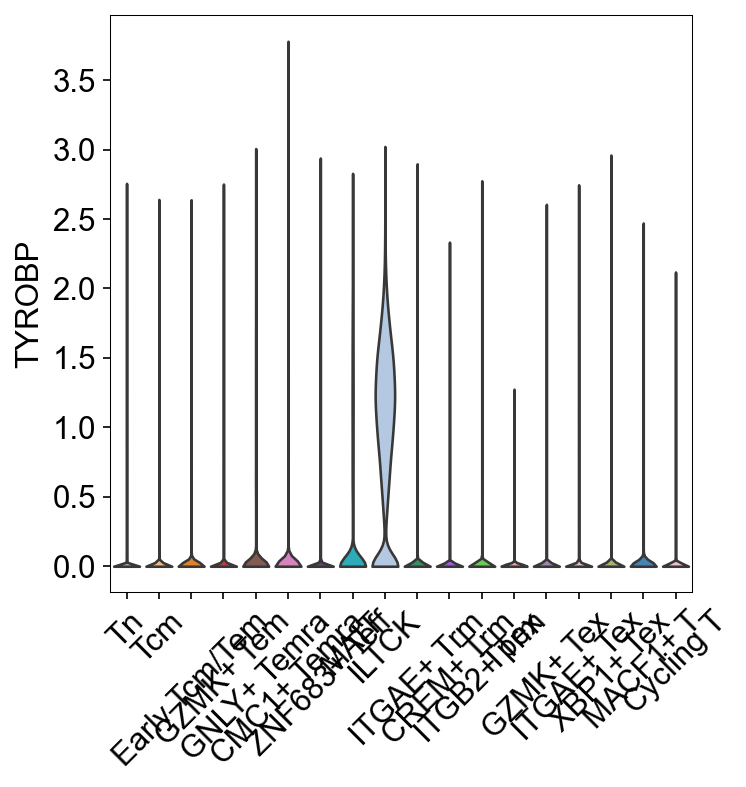
sc.pl.dotplot(adata_gene, var_names=['S1PR1', 'LEF1', 'SELL', 'IL7R',
'KLRG1', 'GZMK', 'CX3CR1', 'GZMB', 'NKG7',
'GNLY', 'CMC1', 'ZNF683',
'KLRB1', 'ZBTB16', 'FCER1G', 'TYROBP',
'ITGAE', 'ITGA1', 'CREM', 'ITGB2',
'TCF7', 'PDCD1', 'CTLA4',
'LAG3', 'HAVCR2',
'CXCR6', 'TIGIT', 'XBP1',
'MACF1', 'MKI67', 'CDC20'],
groupby='cell_subtype_3', dot_max=0.7, ax=ax,
standard_scale='var')
fig.savefig('figures/Figure2B_marker_genes.pdf')
TCR-repertoire Features¶
## D50 cc_aa_identity, Figure1E
kid_studies = ['Wang_2021a', 'Ramaswamy_2021']
old_sname = ['F020', 'F021', 'F023', 'OH14', 'OH15', 'OH17']
young_sname = ['F016', 'F017', 'F024']
adata_circ_nokid = adata_circ[~adata_circ.obs['study_name'].isin(kid_studies)]
adata_circ_nokid = adata_circ_nokid[~adata_circ_nokid.obs['sample_name'].isin(old_sname+young_sname)]
adata_tissue_TIL_nokid = adata_tissue_TIL[~adata_tissue_TIL.obs['study_name'].isin(kid_studies)]
adata_circ_nokid = adata_circ_nokid[adata_circ_nokid.obs['meta_cell_subset']=='normal_CD8']
adata_tissue_TIL_nokid = adata_tissue_TIL_nokid[adata_tissue_TIL_nokid.obs['meta_cell_subset']=='normal_CD8']
d50_disease = cbind_df(adata_circ_nokid, adata_tissue_TIL_nokid,
xaxis=disease_types, xaxis_label='disease_type', index_type='D50')
multi_boxplot(d50_disease,
xaxis='origin', yaxis='value', hueaxis='variable',
file_name='source/d50_disease.pdf')
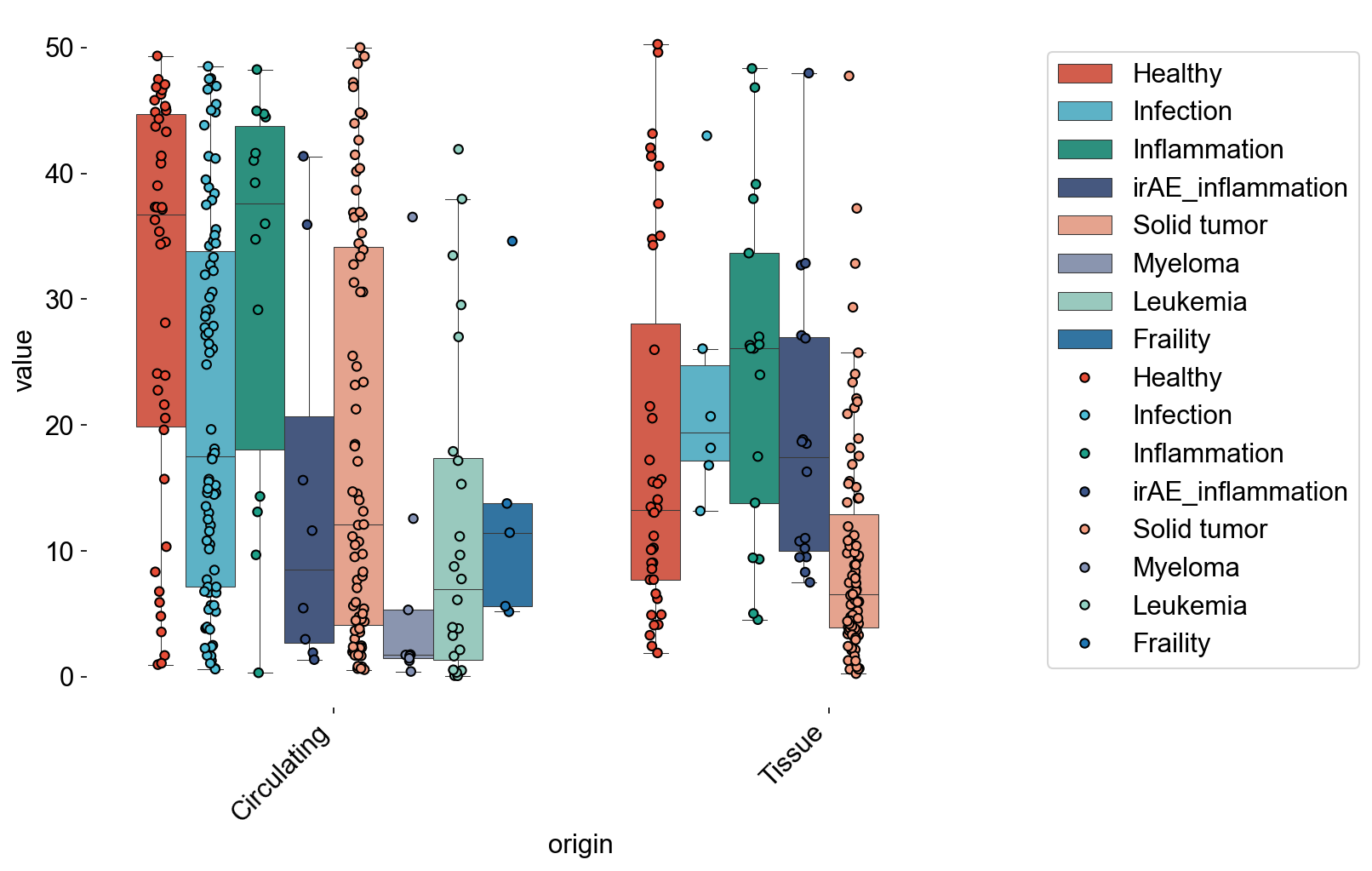
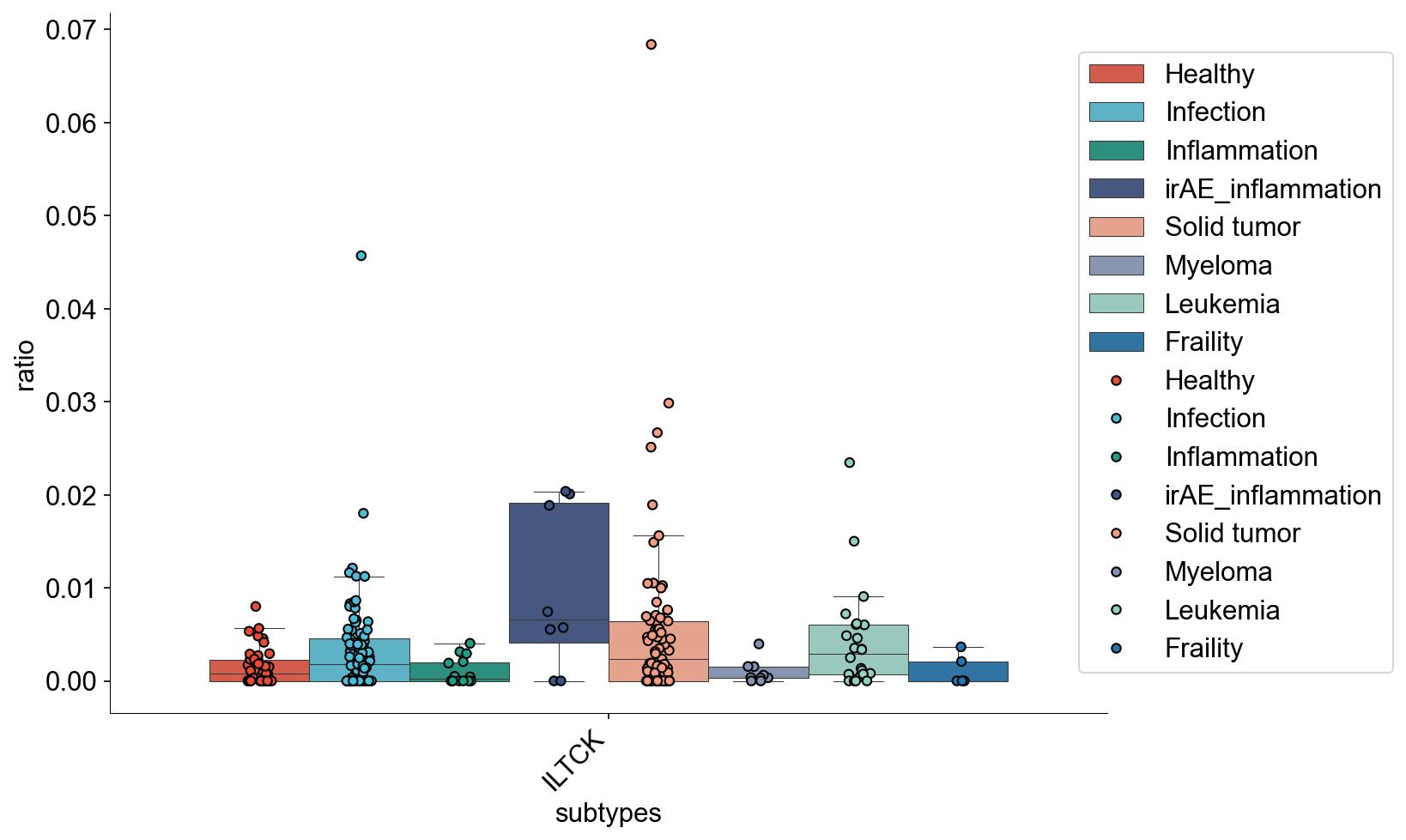
Counter(d50_disease[d50_disease['origin']=='Circulating']['variable'])
Counter({'Healthy': 42,
'Fraility': 5,
'Solid tumor': 75,
'Infection': 83,
'Inflammation': 14,
'Leukemia': 24,
'Myeloma': 9,
'irAE_inflammation': 8})
d50_disease_circ = d50_disease[d50_disease['origin']=='Circulating']
d50_disease_tissue = d50_disease[d50_disease['origin']=='Tissue']
stats.mannwhitneyu(d50_disease_circ[d50_disease_circ['variable']=='Healthy'].value,
d50_disease_circ[d50_disease_circ['variable']=='Fraility'].value)
MannwhitneyuResult(statistic=163.0, pvalue=0.04483359507777036)
## Figure1F, stats of circos plot
clone_cross = clone_subtype[(clone_subtype['study_name'].isin(circ_tissue))]
general_share = dict(Counter(clone_cross['sharing_type']))
general_share_df = pd.DataFrame.from_dict(general_share, orient='index')
general_share_df = general_share_df[general_share_df[0]>=5]
general_share_df.to_csv('figures/Figure2i.csv')
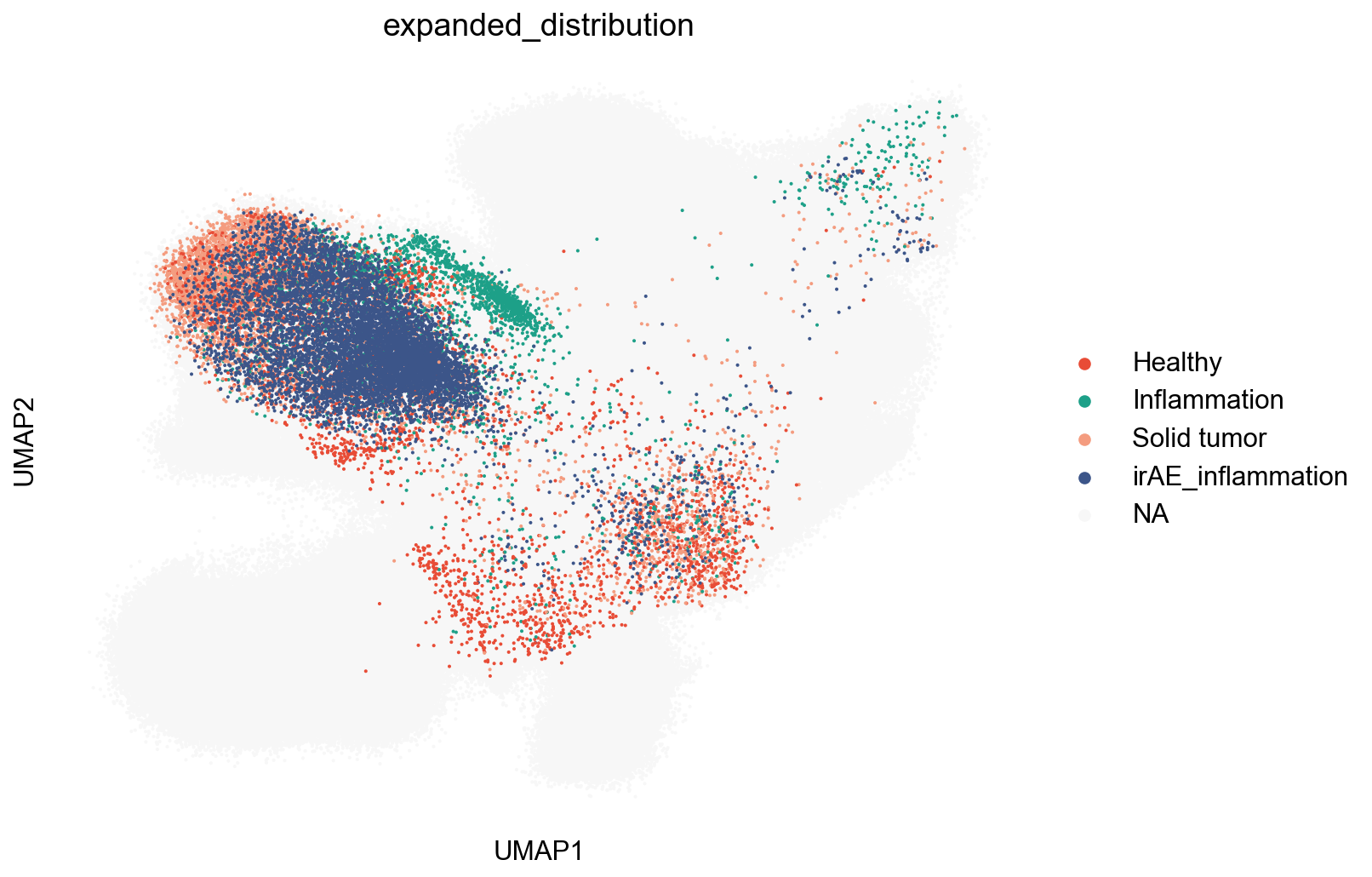
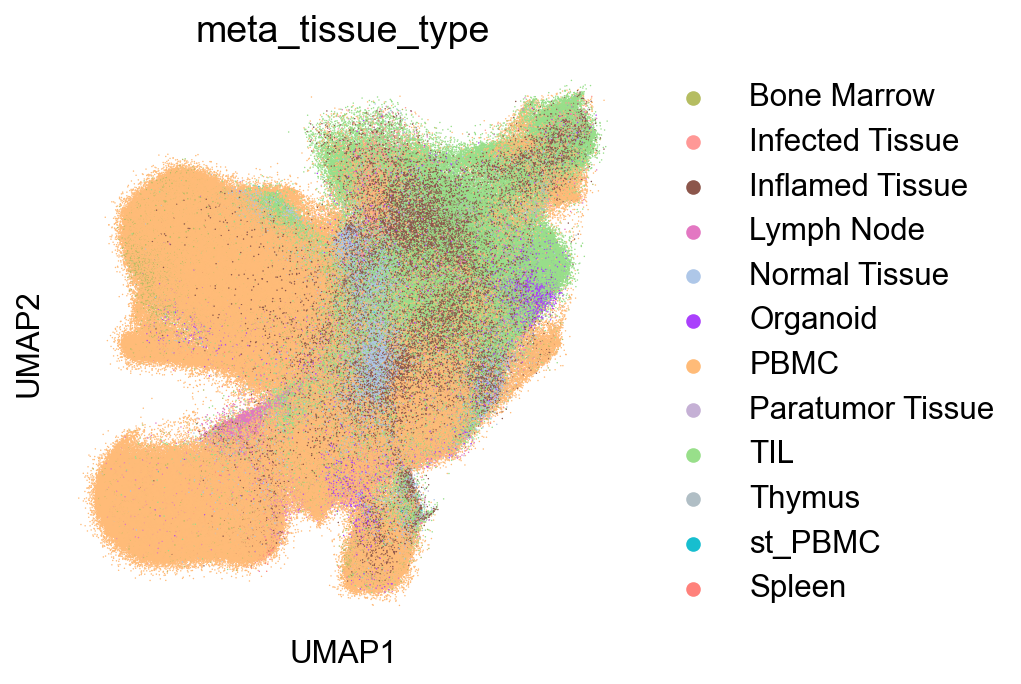
SourceDistribution¶
adata_nmal = adata_nogex[adata_nogex.obs['meta_cell_subset']=='normal_CD8']
## figure1f
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nmal, color='meta_tissue_type',
palette=tissue_type_color, size=2, ax=ax)
fig.savefig('figures/meta_tissue_type.png')
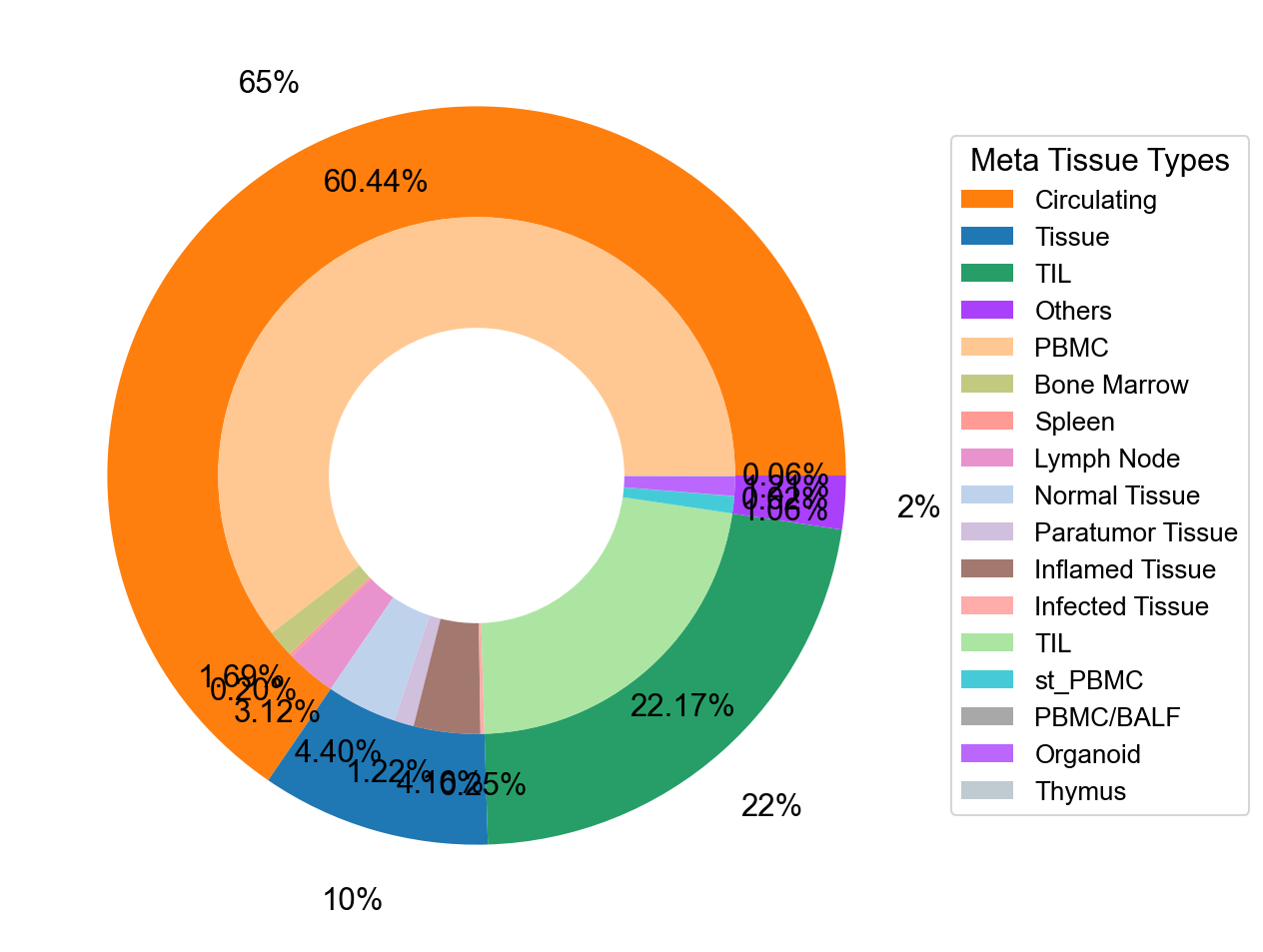
## Supfigure1g, pie chart
tissue_subpercent = []
for j in meta_tissue_type_aggrs:
cell_num = [len(adata_nmal.obs[(adata_nmal.obs['meta_tissue_type_aggr']==j) &
(adata_nmal.obs['meta_tissue_type']==i)]) for i in meta_tissue_types]
tissue_subpercent.append(cell_num)
tissue_subpercent = np.array(tissue_subpercent)
fig,ax = plt.subplots(figsize=(10,8))
color_map = meta_tissue_type_color.values()
inner_color_map = tissue_type_color.values()
ax.pie(tissue_subpercent.sum(axis=1), radius=1,
colors=color_map,
autopct='%1.0f%%', pctdistance=1.2,
wedgeprops=dict(width=0.3))
ax.pie(tissue_subpercent.flatten()[tissue_subpercent.flatten()!=0], radius=0.7,
colors=inner_color_map,
autopct='%1.2f%%', pctdistance=1.2,
wedgeprops=dict(width=0.3, alpha=0.8))
ax.legend(list(meta_tissue_type_color.keys())+list(tissue_type_color.keys()),
title="Meta Tissue Types",
loc="center left",
fontsize="small",
bbox_to_anchor=(1,0,0.5,1))
fig.savefig('figures/tissue_subpercent.pdf')
plt.show()
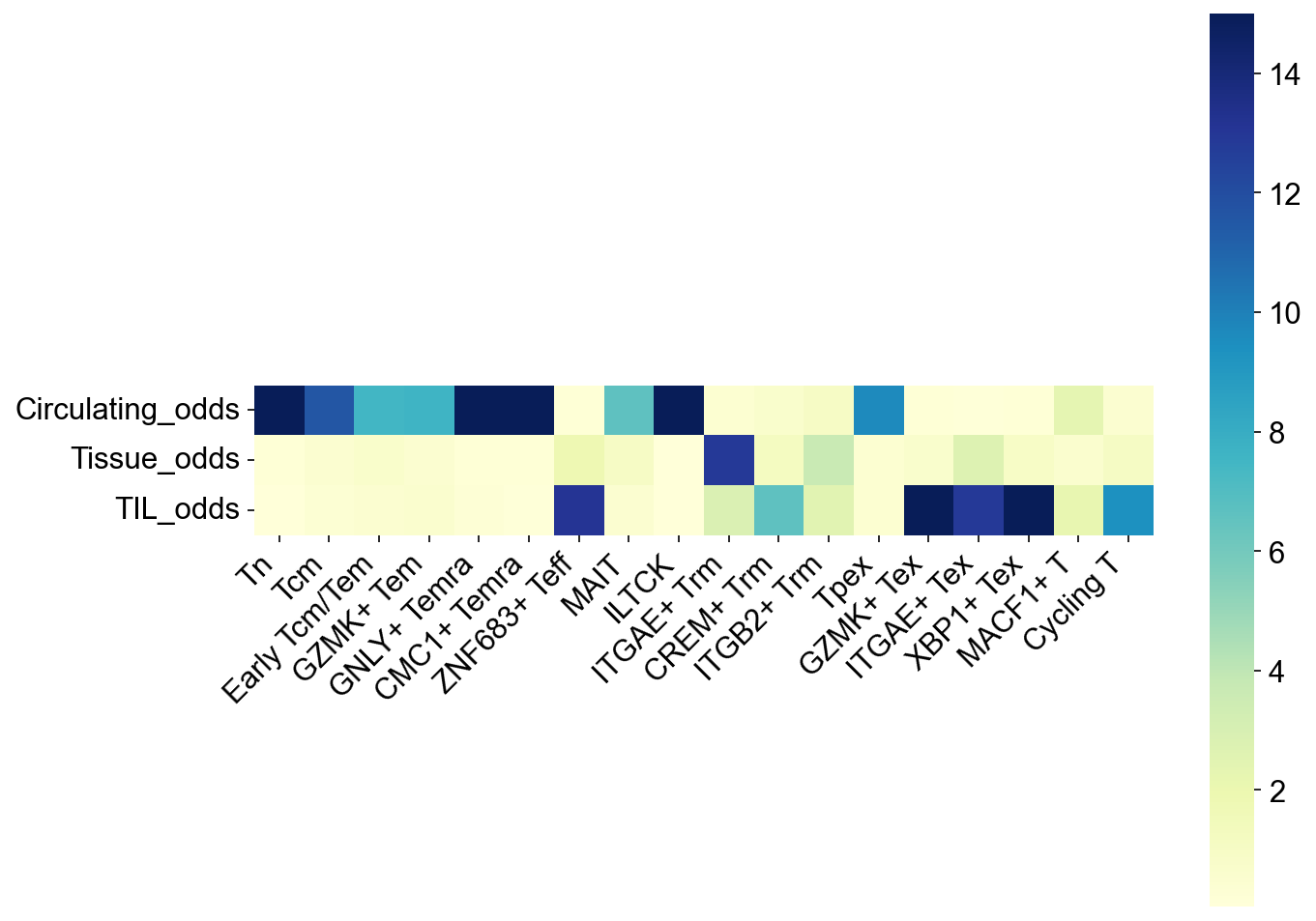
## Figure2H
tissue_subtype_odds = odds_ratio_df(
adata=adata_nmal,
groupby='meta_tissue_type_aggr', groups=meta_tissue_type_aggrs[:3])
fig,ax = plt.subplots(figsize=(10,8))
ax = sns.heatmap(tissue_subtype_odds.T, square=True, cmap='YlGnBu', vmax=15)
ax.set_xticklabels(ax.get_xticklabels(), rotation=45, horizontalalignment='right')
fig.savefig('figures/Figure2e_subtype_odds.pdf')
plt.show()
SupTables¶
## stats
adata_nogex.uns['log1p']['base'] = None
sc.tl.rank_genes_groups(adata_nogex, groupby='cell_subtype_3')
WARNING: Default of the method has been changed to 't-test' from 't-test_overestim_var'
subtype_sheet_names = [
'Tn',
'Tcm',
'EarlyTcmTem',
'GZMK+Tem',
'GNLY+Temra',
'CMC1+Temra',
'ZNF683+Teff',
'MAIT',
'ILTCK',
'ITGAE+Trm',
'CREM+Tm',
'ITGB2+Trm',
'Tpex',
'GZMK+Tex',
'ITGAE+Tex',
'XBP1+Tex',
'MACF1+T',
'CyclingT'
]
with pd.ExcelWriter('tables/marker_genes.xls') as marker_excel:
for i,j in zip(cell_subtypes,subtype_sheet_names):
subtype_marker = sc.get.rank_genes_groups_df(adata_nogex, group=i)
subtype_marker = subtype_marker[~subtype_marker['names'].str.startswith('RP') &
~subtype_marker['names'].str.startswith('MT-')]
subtype_marker.to_excel(marker_excel, sheet_name=j, index=False)
sample_info = adata_nogex.obs.drop_duplicates(subset='sample_name')
sample_info.index = sample_info['sample_name']
sample_info['cell_num'] = Counter(adata_nogex.obs['sample_name'])
sample_info = sample_info[['study_name','individual_id',
'meta_tissue_type','meta_tissue_type_aggr',
'cell_subset','meta_cell_subset','disease','disease_type',
'treatment', 'treatment_status', 'donor_sex','donor_age']]
sample_info = sample_info.sort_values(['study_name', 'sample_name', 'meta_tissue_type_aggr'])
sample_info.to_excel('tables/sample_info.xls', index=True)
SupFigure7¶
Extended Data Figure 7A¶
## Supfigure1A
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='study_name', size=2, ax=ax)
fig.savefig('figures/S7A_study_name.png')
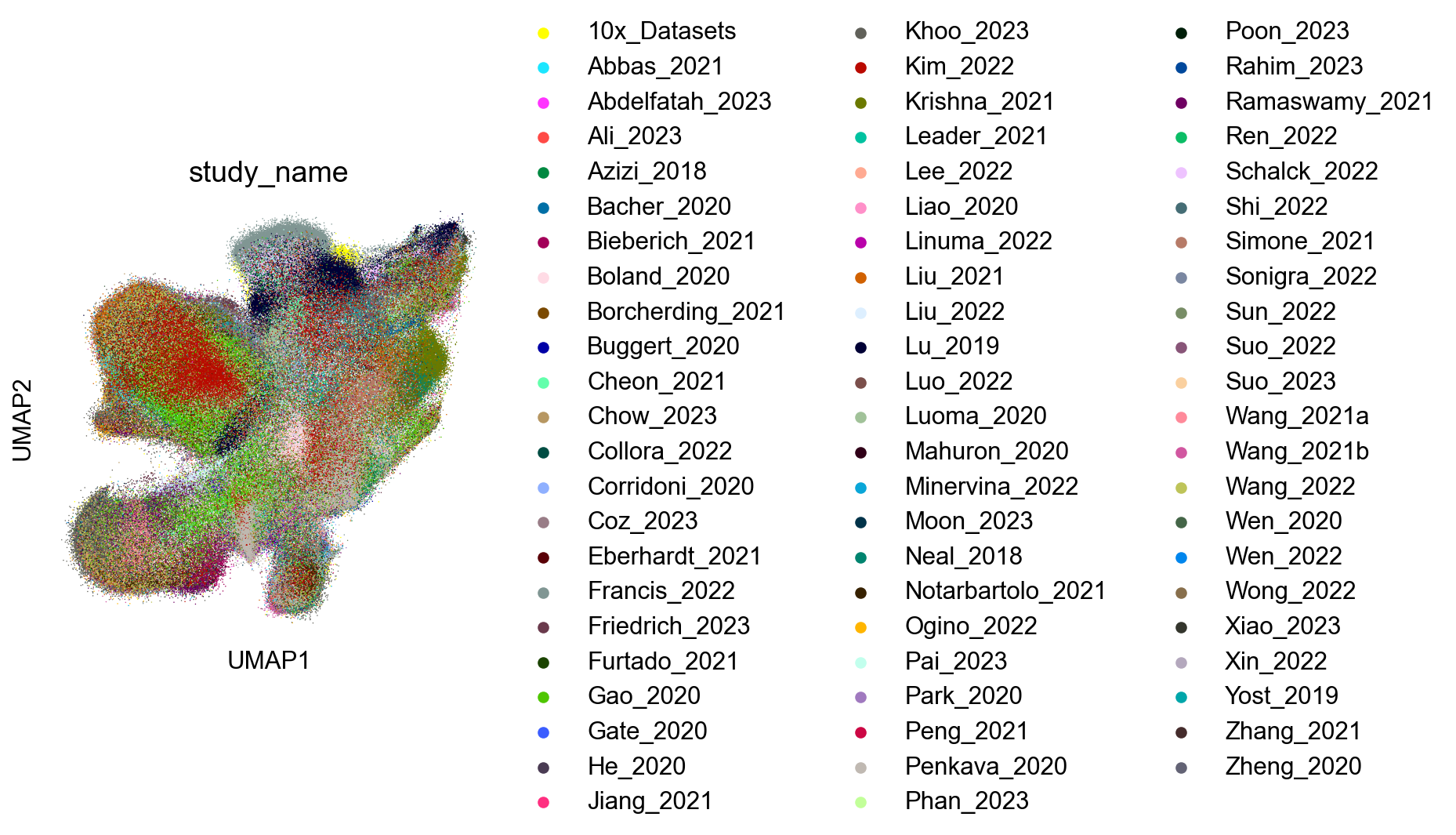
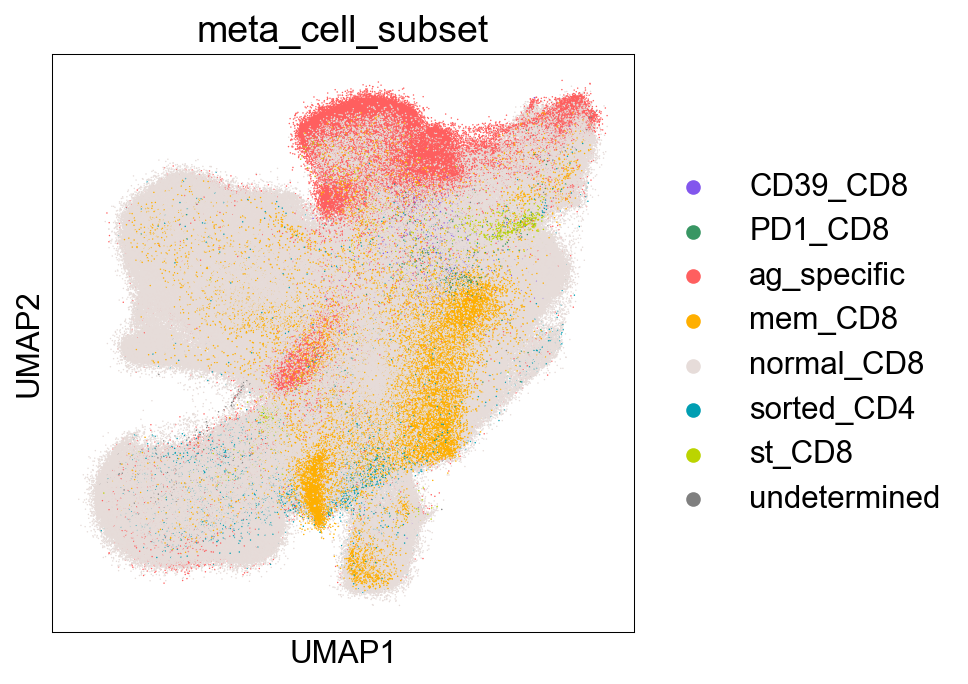
Extended Data Figure 7B-D (Sorting)¶
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='meta_cell_subset', size=2,
palette=sorting_color, ax=ax)
fig.savefig('figures/S7B_meta_cell_subset.png')
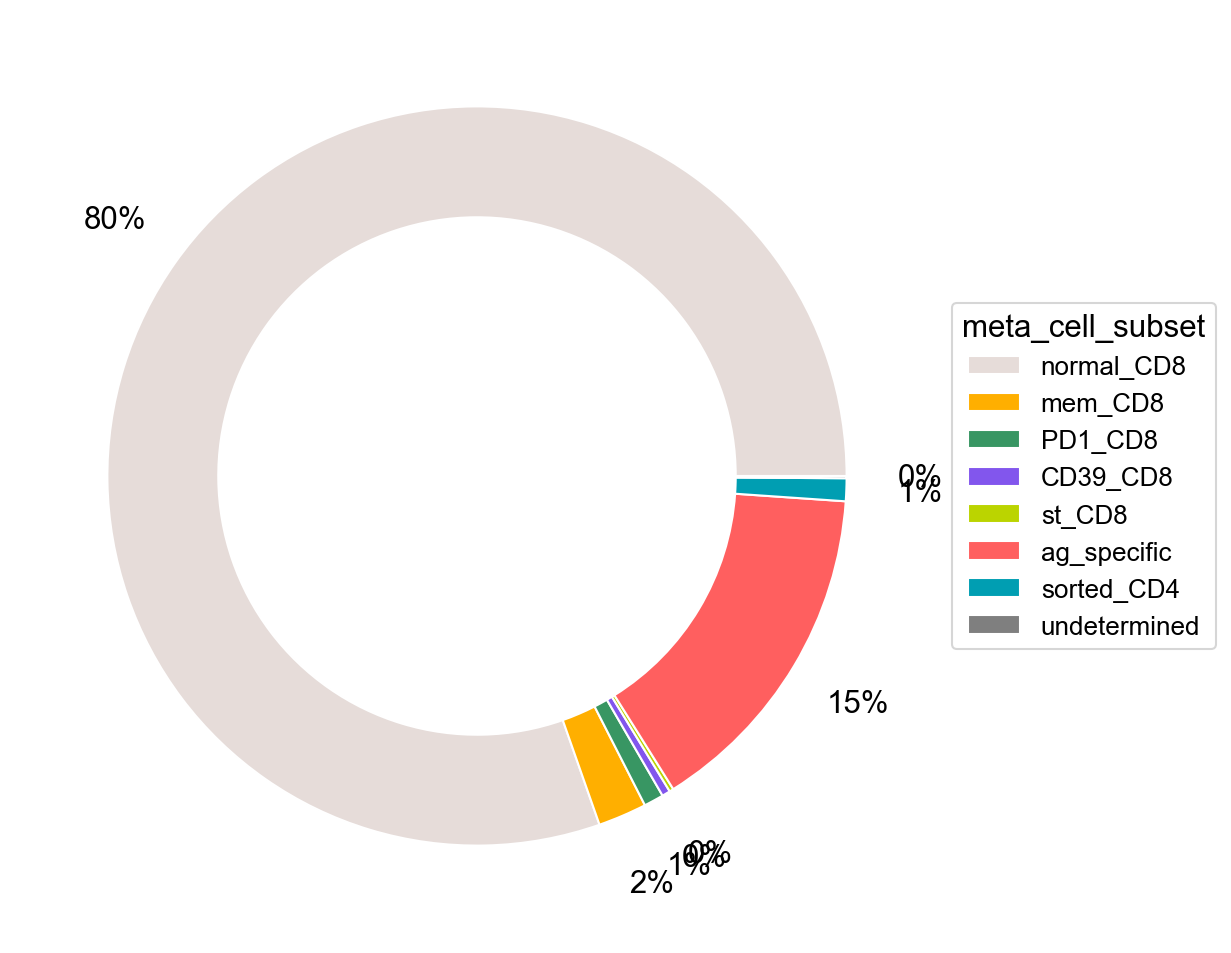
pie_plot(adata_nogex,
items=meta_cell_subsets,
item_str='meta_cell_subset',
item_color=sorting_color,
file_name='figures/S7_subset_percent.pdf')
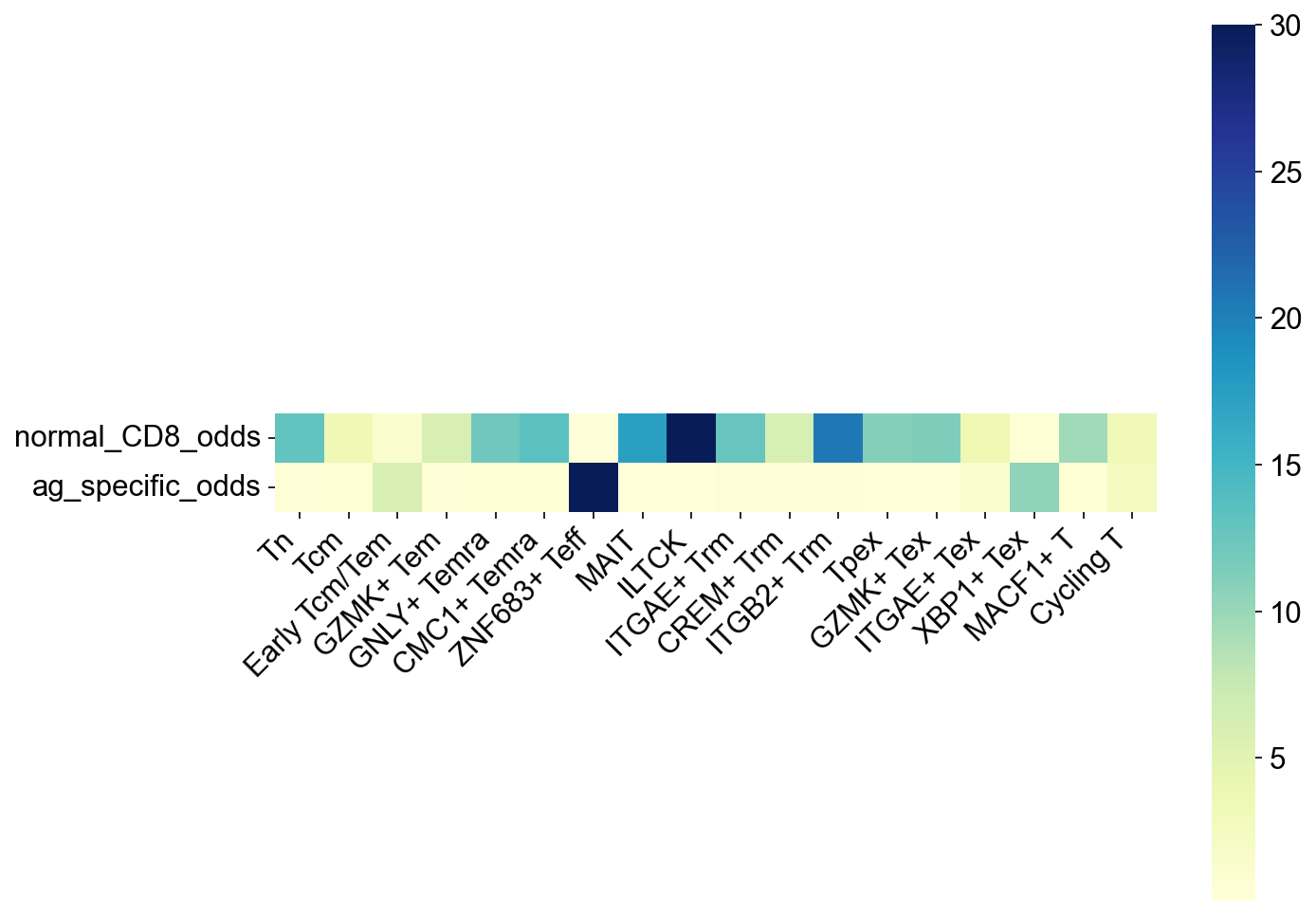
facs_subset = ['normal_CD8_odds', 'ag_specific_odds',]
facs_odds = odds_ratio_df(groupby='meta_cell_subset', groups=meta_cell_subsets,subsets=facs_subset)
fig,ax = plt.subplots(figsize=(10,8))
ax = sns.heatmap(facs_odds.T, square=True, cmap='YlGnBu', vmax=30)
ax.set_xticklabels(ax.get_xticklabels(), rotation=45, horizontalalignment='right')
fig.savefig('figures/S7_cell_subset_odds.pdf')
plt.show()
Extended Data Figure 7E-G (Disease)¶
## Supfigure1D
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='disease_type', palette=disease_type_color, size=2, ax=ax)
fig.savefig('figures/S7_disease_type.pdf')
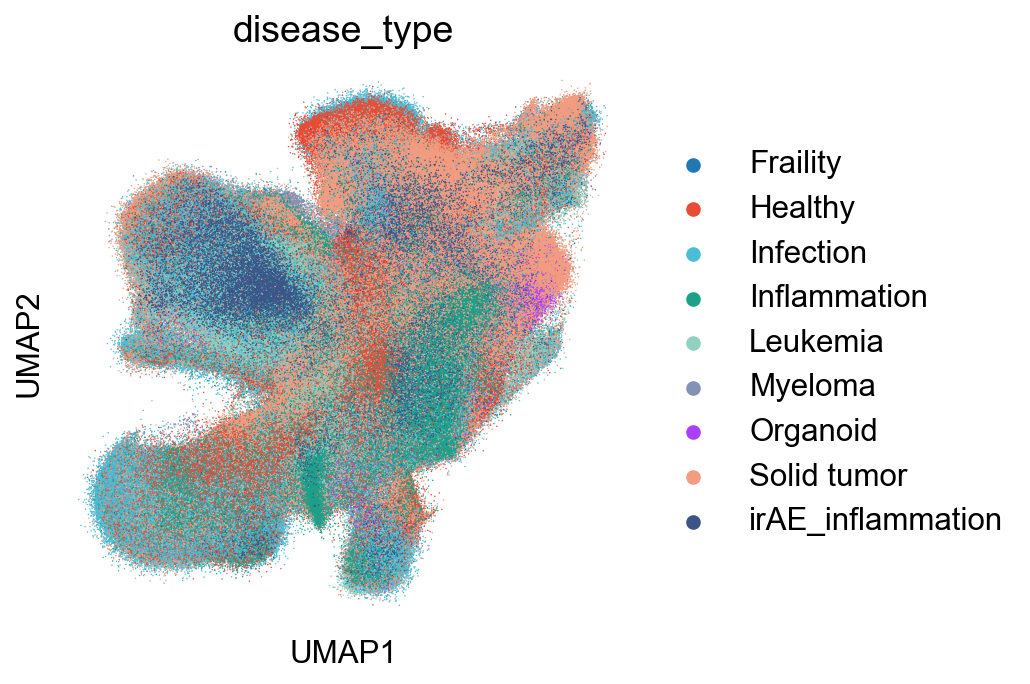
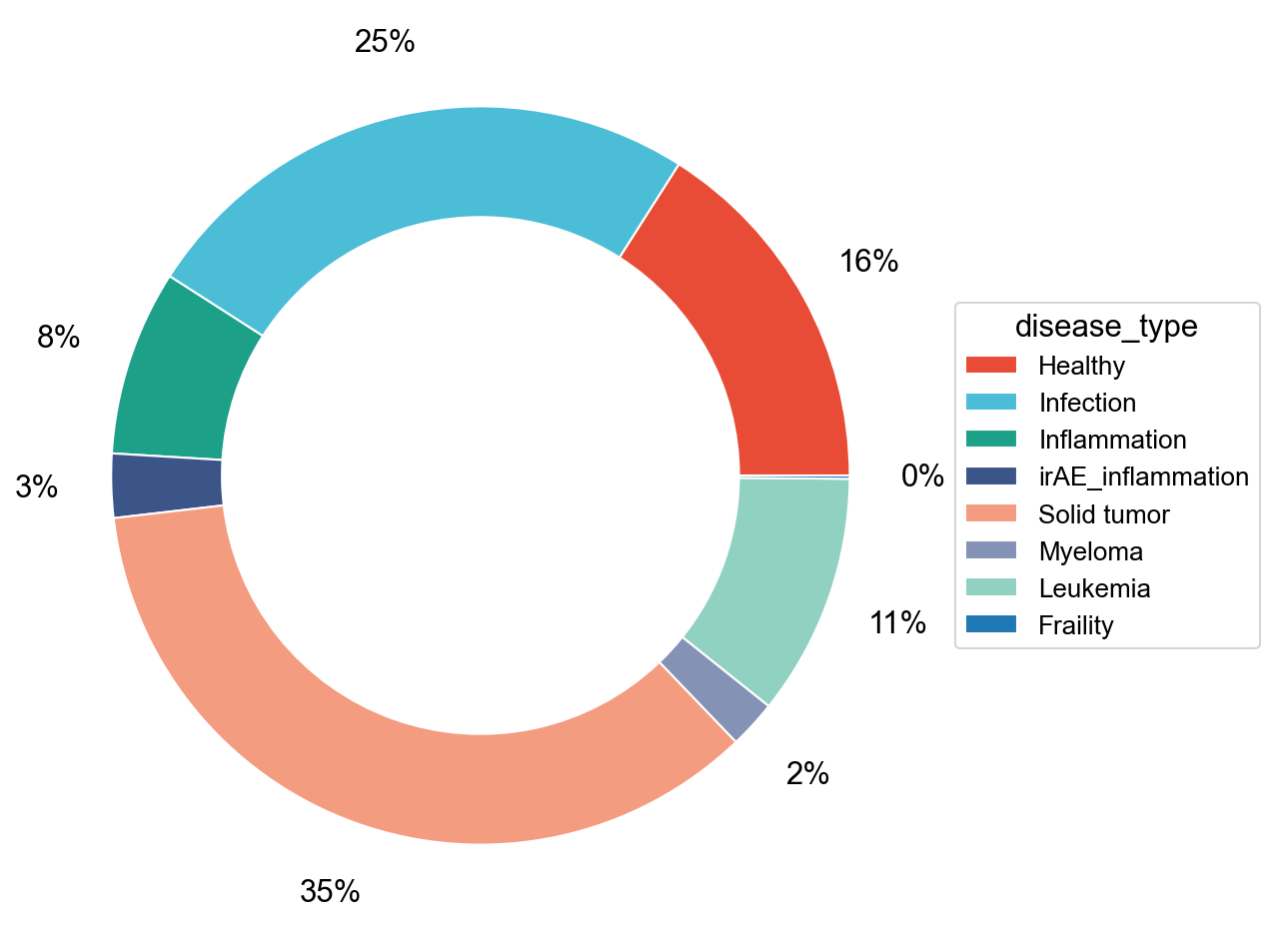
## Supfigure1D
pie_plot(adata_nogex,
items=disease_types,
item_str='disease_type',
item_color=disease_type_color,
file_name='figures/S7_condition_percent.pdf')
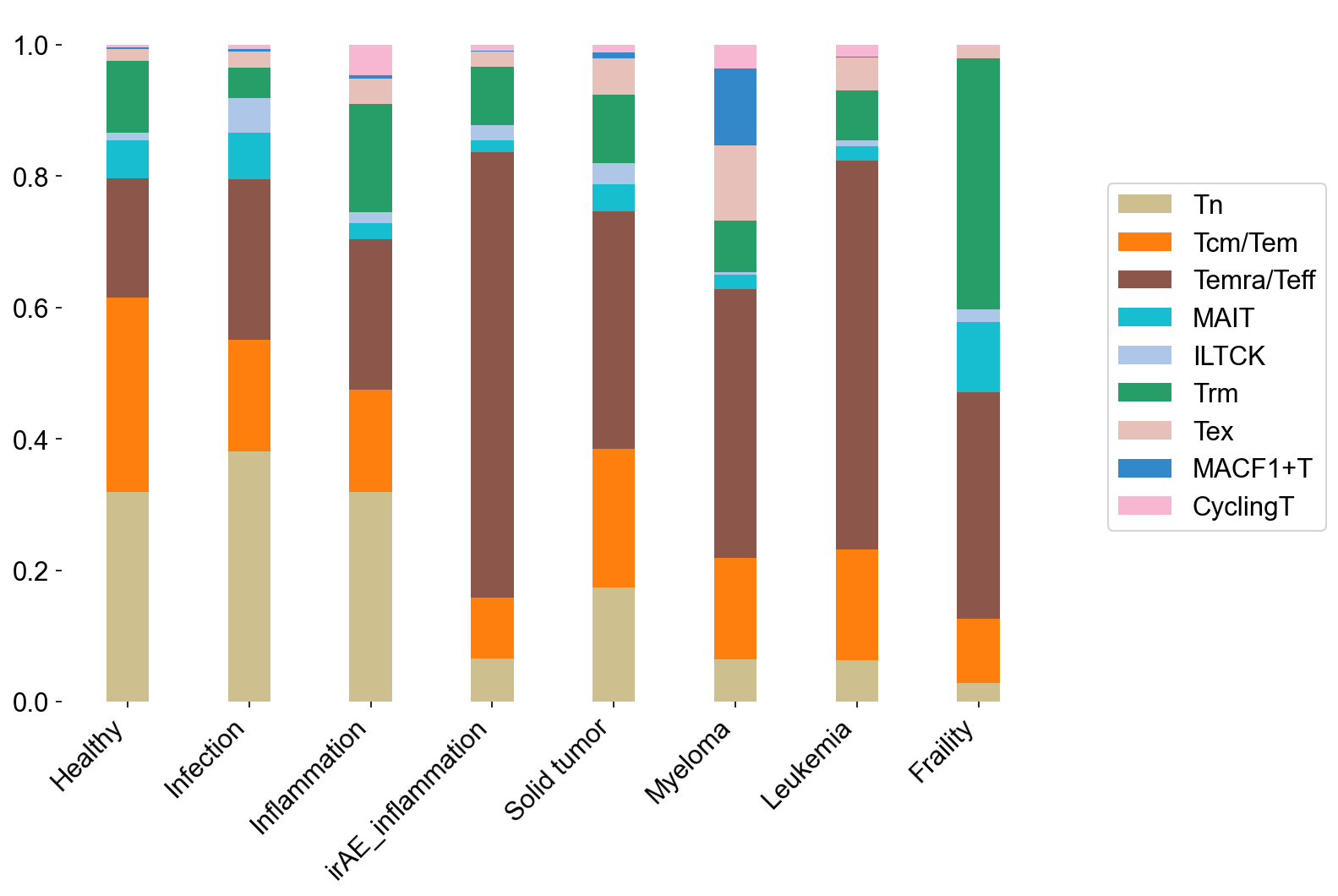
stacked_bar(adata=adata_circ_nokid[adata_circ_nokid.obs['meta_cell_subset']=='normal_CD8'],
percentage=True, xitems=disease_types, xitem_str='disease_type',
yitems=cell_subtype_aggrs, yitem_str='meta_cell_subtype', yitem_color=main_type_color,
file_name='figures/S7_disease2subtypes_circ.pdf')
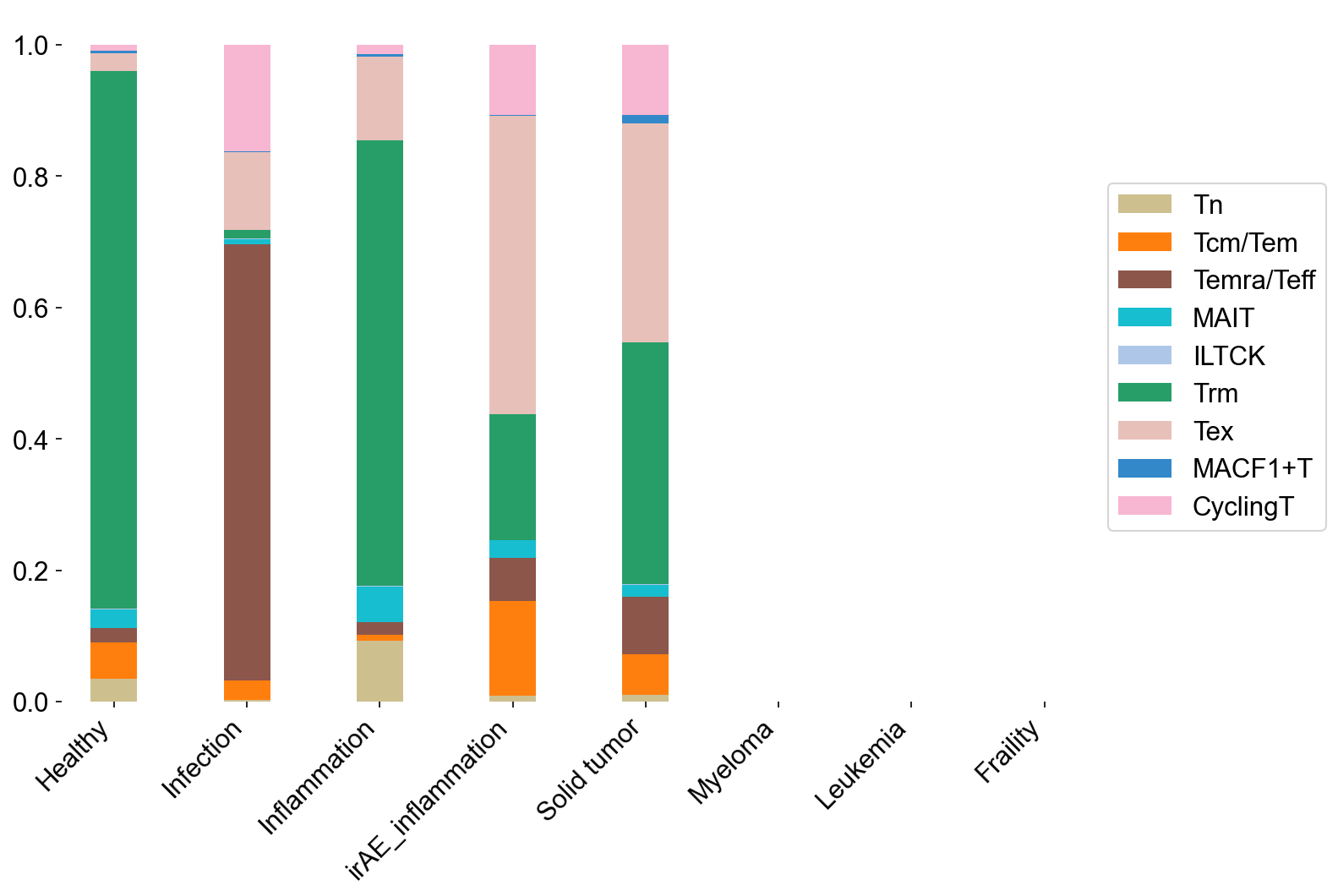
stacked_bar(adata=adata_tissue_TIL_nokid[adata_tissue_TIL_nokid.obs['meta_cell_subset']=='normal_CD8'],
percentage=True, xitems=disease_types, xitem_str='disease_type',
yitems=cell_subtype_aggrs, yitem_str='meta_cell_subtype', yitem_color=main_type_color,
file_name='figures/S7_disease2subtypes_tissue.pdf')
## SupFigureS6G
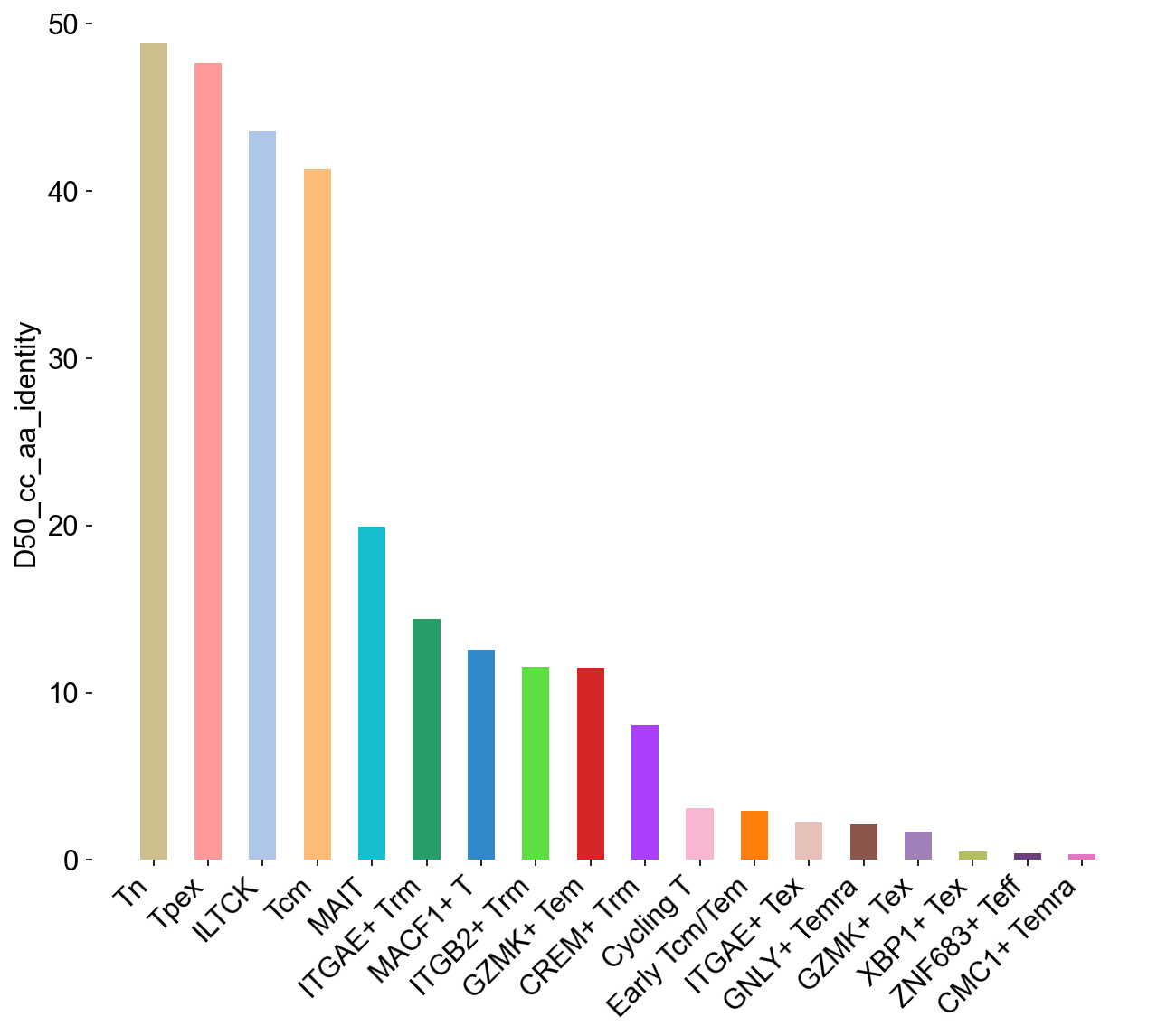
ir.tl.alpha_diversity(adata_nogex, groupby='cell_subtype_3', target_col='cc_aa_identity', metric='D50')
adata_nogex.obs['D50_cc_aa_identity'] = adata_nogex.obs['D50_cc_aa_identity'].astype(float)
D50_subtypes = adata_nogex.obs[['cell_subtype_3','D50_cc_aa_identity']].drop_duplicates(subset='cell_subtype_3')
D50_subtypes.index = D50_subtypes['cell_subtype_3']
D50_subtypes = D50_subtypes.reindex(cell_subtypes)
D50_subtypes['colors'] = subtype_color.values()
general_bar(D50_subtypes, target_col='D50_cc_aa_identity', ylim0=0, ylim1=50,
file_name='figures/S7_d50_subtype.pdf')
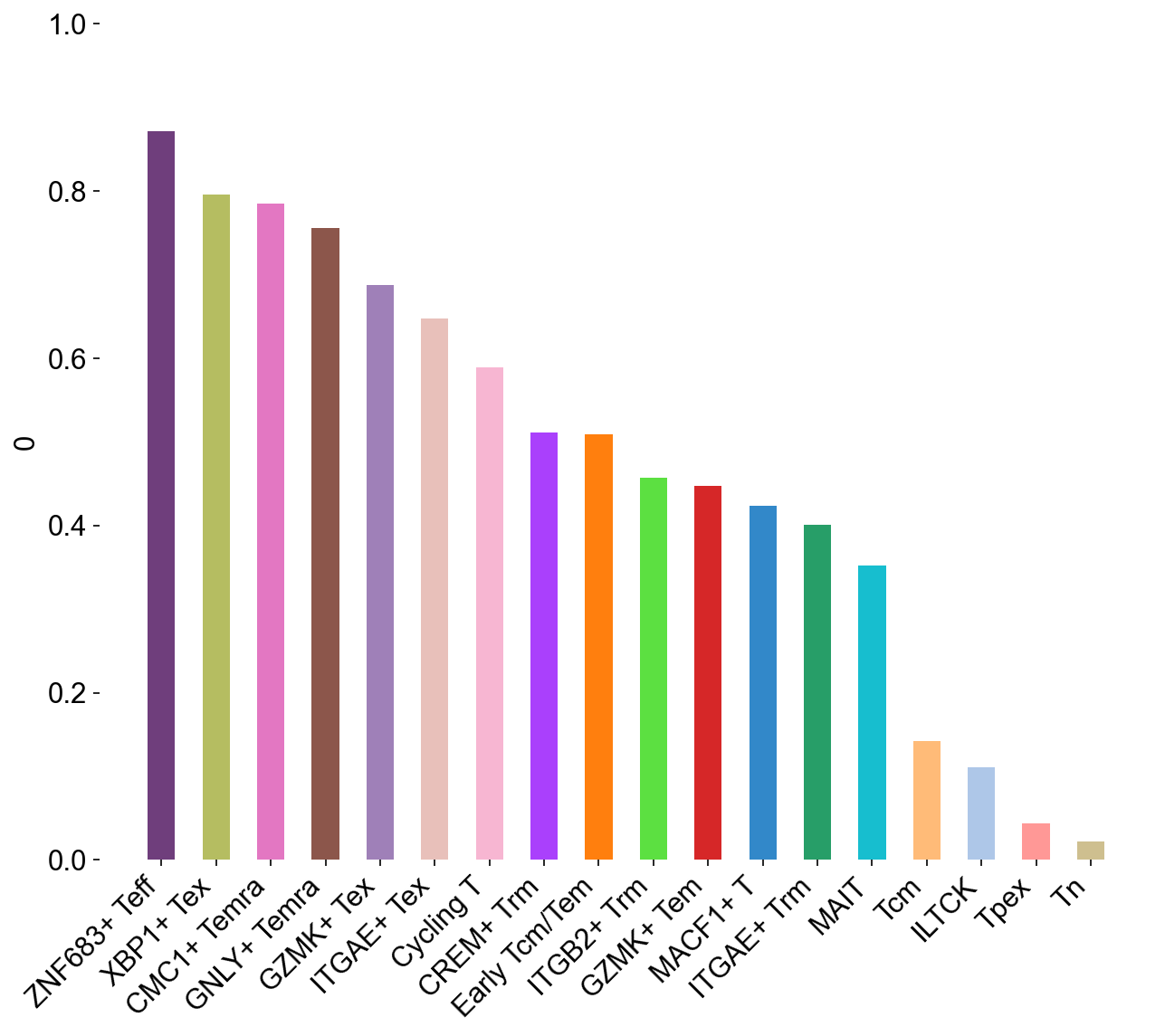
## SupFigureS6H
clone_group=[]
for i in cell_subtypes:
subgroup_sample = adata_nogex.obs[adata_nogex.obs['cell_subtype_3']==i]
clone_sample = dict(Counter(subgroup_sample.cc_aa_identity))
G = gini(list(clone_sample.values()))
clone_group.append(G)
clone_gini_df = pd.DataFrame(clone_group, index=cell_subtypes)
clone_gini_df['colors'] = subtype_color.values()
general_bar(clone_gini_df, target_col=0, ylim0=0, ylim1=1,
file_name='figures/S7_gini_subtype.pdf')
## SupFigureS6I
gini_disease = cbind_df(adata_circ_nokid, adata_tissue_TIL_nokid,
xaxis=disease_types, xaxis_label='disease_type', index_type='gini')
multi_boxplot(gini_disease, xaxis='origin', hueaxis='variable',
file_name='figures/S7_gini_disease.pdf')
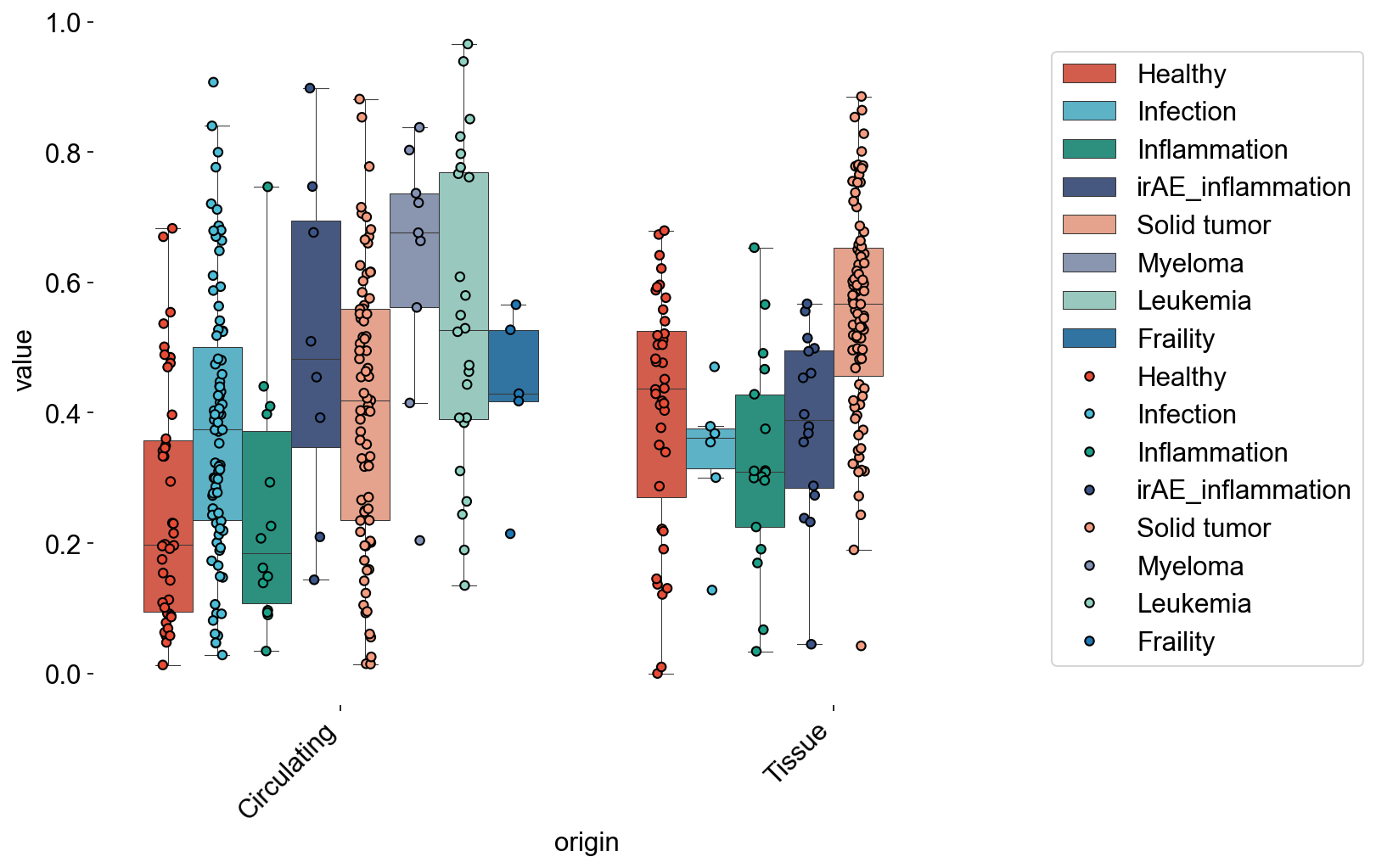
gini_disease_circ = gini_disease[gini_disease['origin']=='Circulating']
gini_disease_tissue = gini_disease[gini_disease['origin']=='Tissue']
stats.mannwhitneyu(gini_disease_circ[gini_disease_circ['variable']=='Healthy'].value,
gini_disease_circ[gini_disease_circ['variable']=='Fraility'].value)
MannwhitneyuResult(statistic=44.0, pvalue=0.033829246143425515)
Part2TwoTypeTexInCancer¶
Functions¶
## blacklist genes which may be affected by dissociation
blacklist = [
"ACTG1",
"BTG1",
"CXCL1",
"DNAJB4",
"ERRFI1",
"H3F3B",
"HSPB1",
"IRF1",
"KLF6",
"MIR22HG",
"NFKBIA",
"PCF11",
"PXDC1",
"SDC4",
"SRF",
"TPM3",
"USP2",
"GADD45G",
"ANKRD1",
"BTG2",
"CYR61",
"DUSP1",
"FAM132B",
"HIPK3",
"HSPH1",
"IRF8",
"KLF9",
"MT1",
"NFKBIZ",
"PDE4B",
"RAP1B",
"SERPINE1",
"SRSF5",
"TPPP3",
"WAC",
"HSPE1",
"ARID5A",
"CCNL1",
"DCN",
"DUSP8",
"FOS",
"HSP90AA1",
"ID3",
"ITPKC",
"LITAF",
"MT2",
"NOP58",
"PER1",
"RASSF1",
"SKIL",
"SRSF7",
"TRA2A",
"ZC3H12A",
"IER5",
"ATF3",
"CCRN4L",
"DDX3X",
"EGR1",
"FOSB",
"HSP90AB1",
"IDI1",
"JUN",
"LMNA",
"MYADM",
"NPPC",
"PHLDA1",
"RHOB",
"SLC10A6",
"STAT3",
"TRA2B",
"ZFAND5",
"KCNE4",
"ATF4",
"CEBPB",
"DDX5",
"EGR2",
"FOSL2",
"HSPA1A",
"IER2",
"JUNB",
"MAFF",
"MYC",
"NR4A1",
"PNP",
"RHOH",
"SLC38A2",
"TAGLN2",
"TRIB1",
"ZFP36",
"BAG3",
"CEBPD",
"DES",
"EIF1",
"GADD45A",
"HSPA1B",
"IER3",
"JUND",
"MAFK",
"MYD88",
"ODC1",
"PNRC1",
"RIPK1",
"SLC41A1",
"TIPARP",
"TUBB4B",
"ZFP36L1",
"BHLHE40",
"CEBPG",
"DNAJA1",
"EIF5",
"GCC1",
"HSPA5",
"IFRD1",
"KLF2",
"MCL1",
"NCKAP5L",
"OSGIN1",
"PPP1CC",
"SAT1",
"SOCS3",
"TNFAIP3",
"TUBB6",
"ZFP36L2",
"BRD2",
"CSRNP1",
"DNAJB1",
"ERF",
"GEM",
"HSPA8",
"IL6",
"KLF4",
"MIDN",
"NCOA7",
"OXNAD1",
"PPP1R15A",
"SBNO2",
"SQSTM1",
"TNFAIP6",
"UBC",
"ZYX"
]
StatsFunctions¶
def DEG_analysis(adata, groupby, query_subtype, reference_subtype, dw=True):
## stats
sc.tl.rank_genes_groups(adata, groupby=groupby, method="t-test", key_added='rank_genes_test',
groups=[query_subtype], reference=reference_subtype)
diff_exp_genes = pd.DataFrame(np.hstack([
np.array(list(map(list, adata.uns["rank_genes_test"]["names"]))),
np.array(list(map(list, adata.uns["rank_genes_test"]['logfoldchanges']))),
np.array(list(map(list, adata.uns["rank_genes_test"]['pvals_adj'])))
]),
columns = list(range(3))
)
diff_exp_genes_up = diff_exp_genes[(~diff_exp_genes[0].isin(blacklist)) &
(diff_exp_genes[1].astype('float')>=0.25) & #1.5
(diff_exp_genes[2].astype('float')<=0.05)] #0.01
diff_exp_genes_dw = diff_exp_genes[(~diff_exp_genes[0].isin(blacklist)) &
(diff_exp_genes[1].astype('float')<=-0.25) &
(diff_exp_genes[2].astype('float')<=0.05)]
def enr_res(adata=adata,
groupby=groupby,
diff_exp_genes=diff_exp_genes_up,
subtype=query_subtype):
ht = sc.pl.dotplot(
adata,
diff_exp_genes.iloc[:,0],
groupby=groupby,
show=False,
return_fig=True
)
size_df = ht.dot_size_df
s = set(size_df.loc[subtype,size_df.loc[subtype] > 0.25].index)
enr_res_df = pd.DataFrame(list(filter(lambda x: x in s, diff_exp_genes.iloc[:,0])))
enr_res_use = gseapy.enrichr(gene_list=enr_res_df.iloc[:,0],
organism='Human',
gene_sets=['GO_Biological_Process_2021'])
return enr_res_use
enr_res_up = enr_res(diff_exp_genes=diff_exp_genes_up, subtype=query_subtype)
if dw==True:
enr_res_dw = enr_res(diff_exp_genes=diff_exp_genes_dw, subtype=reference_subtype)
else:
enr_res_dw = enr_res_up
return diff_exp_genes,diff_exp_genes_up,diff_exp_genes_dw,enr_res_up,enr_res_dw
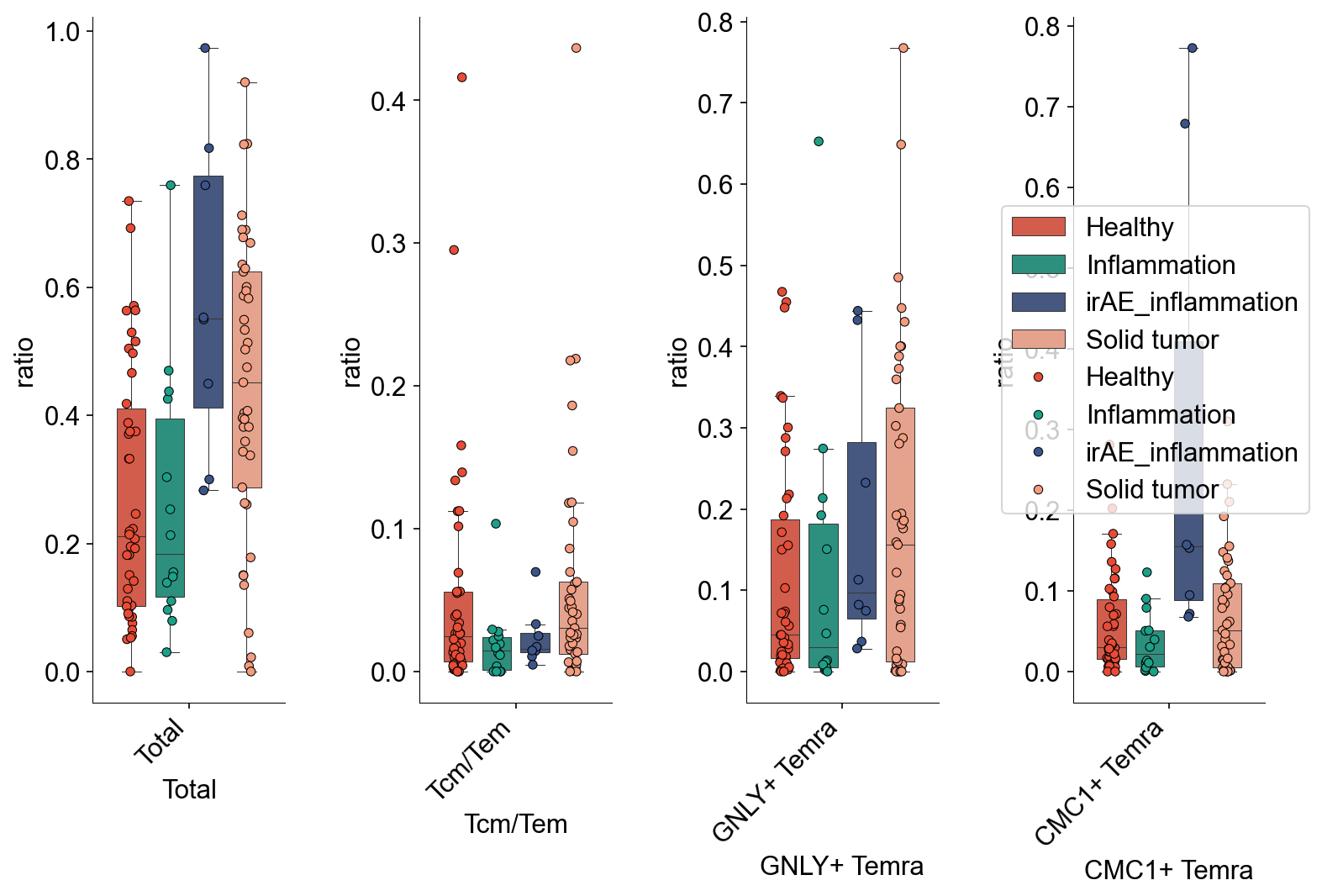
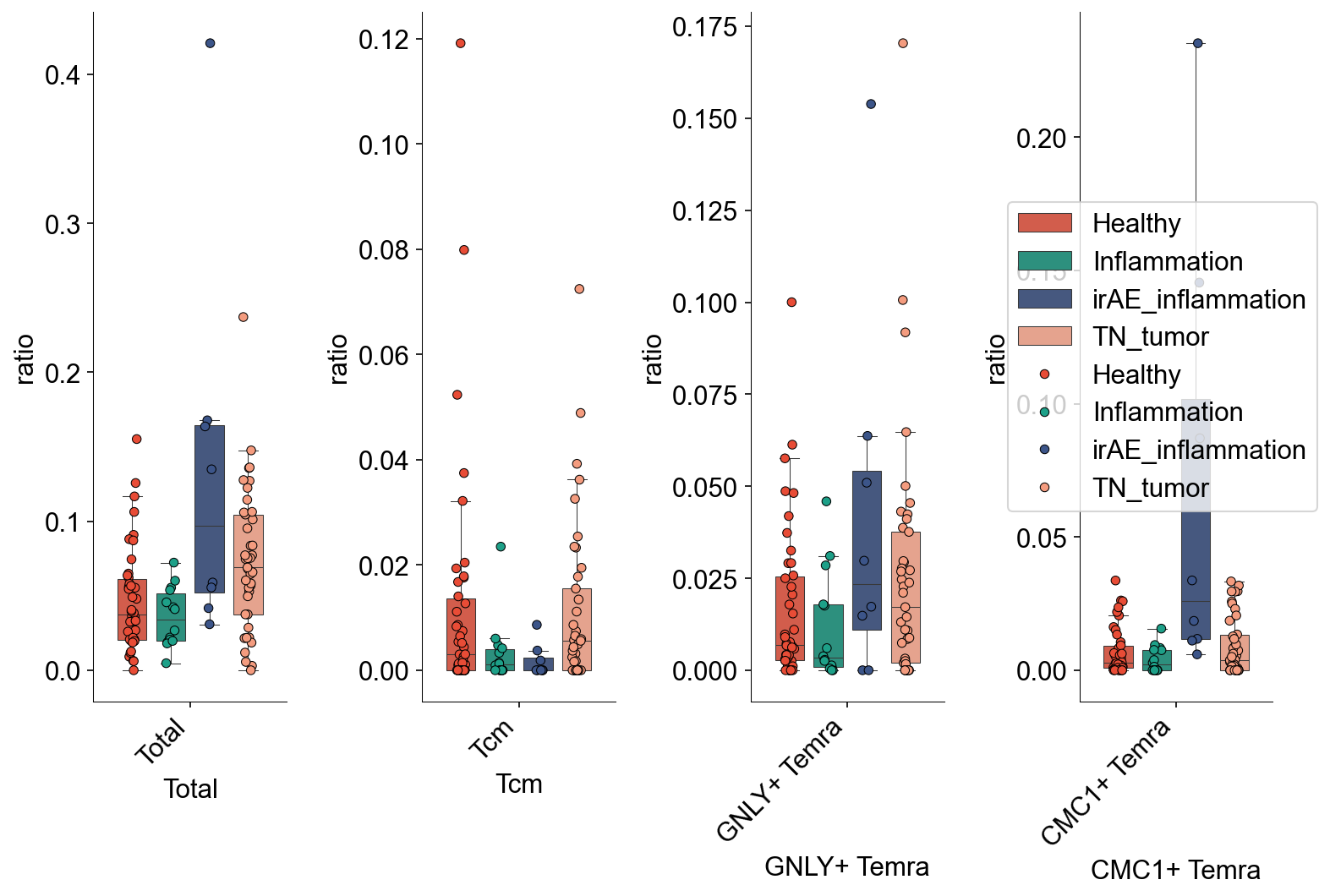
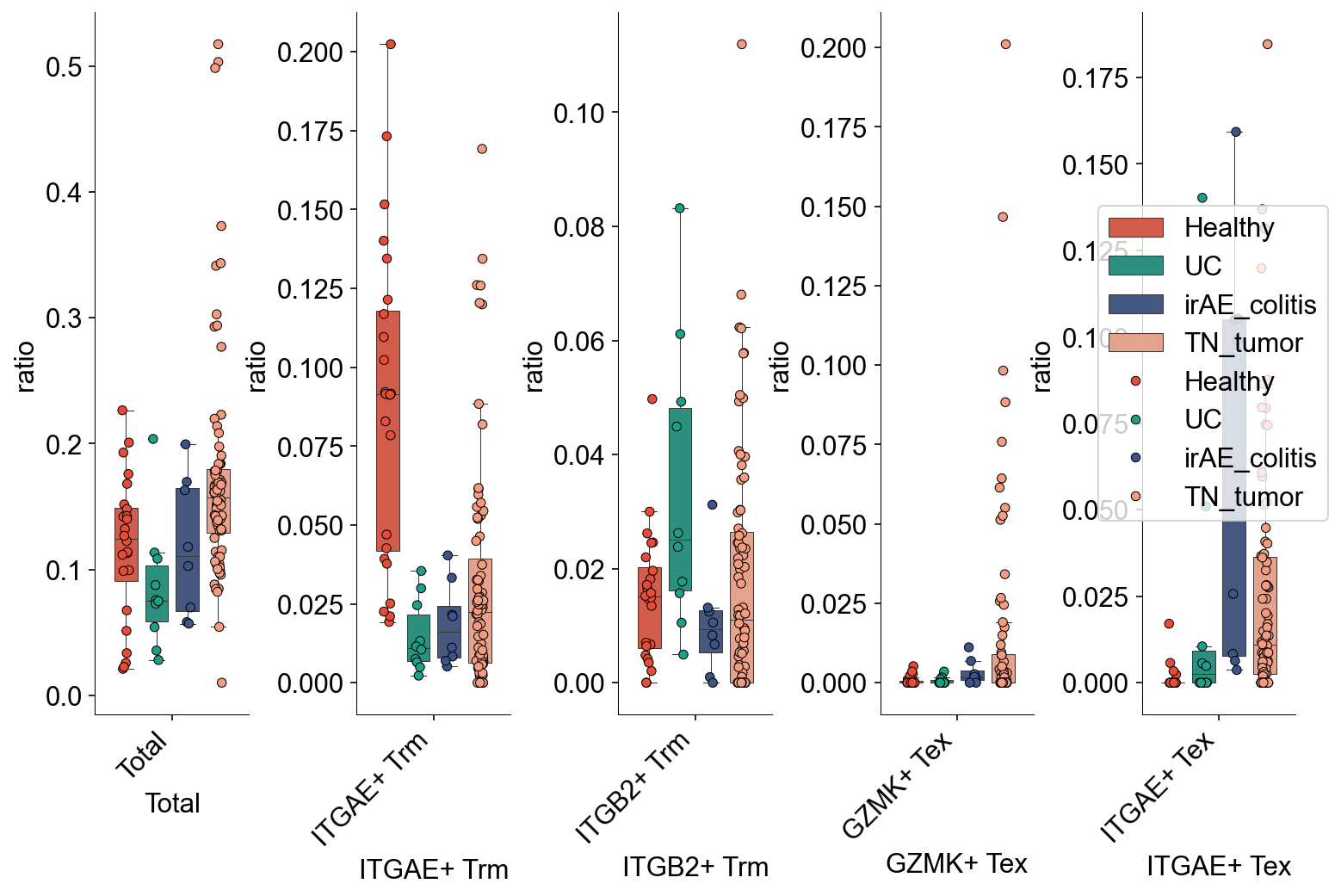
def condition_cell_ratio(adata0, source, cell_type1, cell_type2, cell_type3=None):
adata0 = adata0[adata0.obs['meta_tissue_type_aggr']==source]
a1 = pd.Series(Counter(adata0.obs[adata0.obs["cell_subtype_3"]==cell_type1].sample_name))
b1 = pd.Series(Counter(adata0.obs.sample_name))
x1 = pd.DataFrame({"ratio":a1/b1})
x1 = x1.fillna(0)
a2 = pd.Series(Counter(adata0.obs[adata0.obs["cell_subtype_3"]==cell_type2].sample_name))
b2 = pd.Series(Counter(adata0.obs.sample_name))
x2 = pd.DataFrame({"ratio":a2/b2})
x2 = x2.fillna(0)
x0 = pd.concat([x1,x2])
x0['subtype'] = (
list(np.repeat(cell_type1, len(x1))) +
list(np.repeat(cell_type2, len(x2)))
)
if cell_type3 is not None:
a3 = pd.Series(Counter(adata0.obs[adata0.obs["cell_subtype_3"]==cell_type3].sample_name))
b3 = pd.Series(Counter(adata0.obs.sample_name))
x3 = pd.DataFrame({"ratio":a3/b3})
x3 = x3.fillna(0)
x0 = pd.concat([x0,x3])
x0['subtype'] = (
list(np.repeat(cell_type1, len(x1))) +
list(np.repeat(cell_type2, len(x2))) +
list(np.repeat(cell_type3, len(x3)))
)
return x0
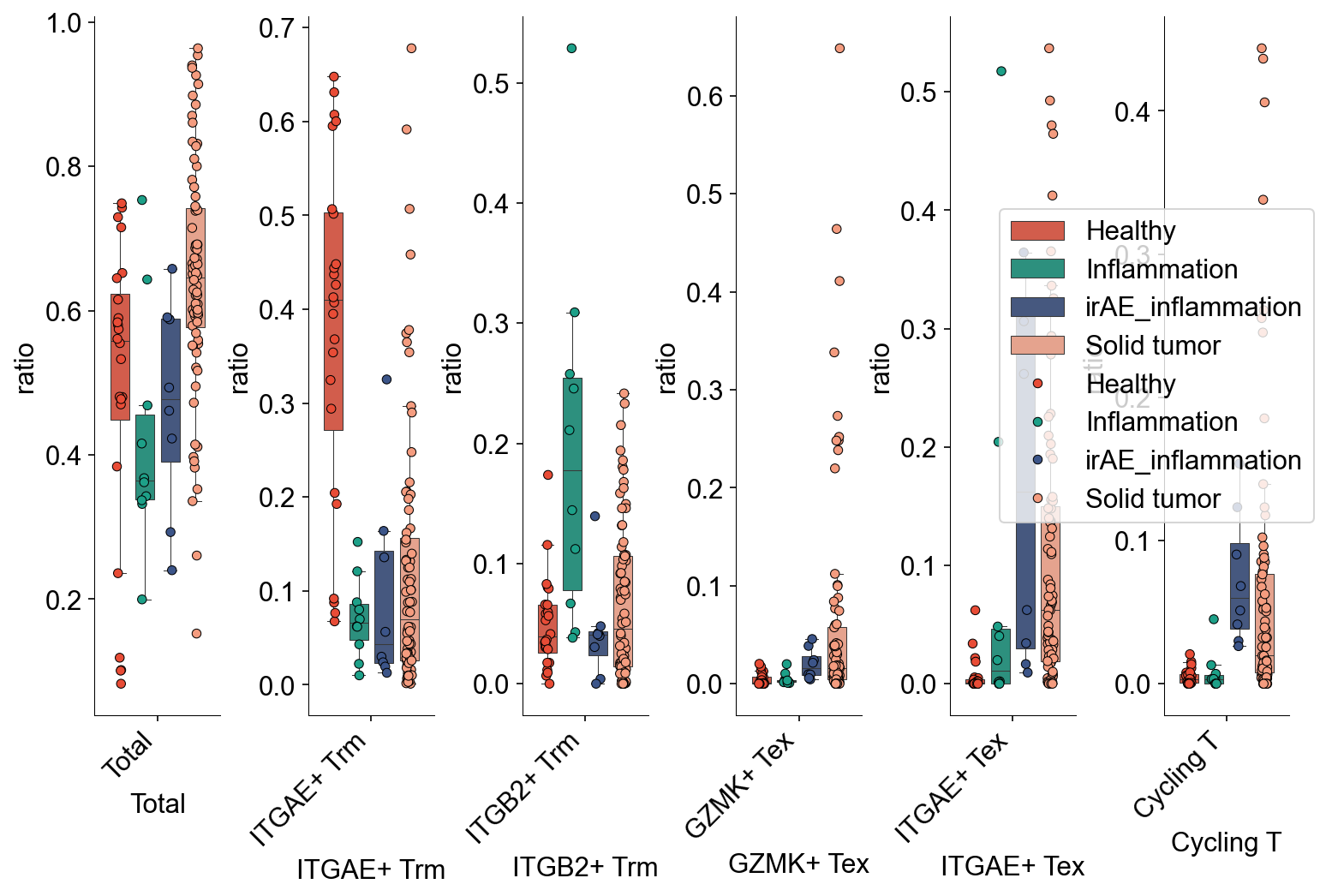
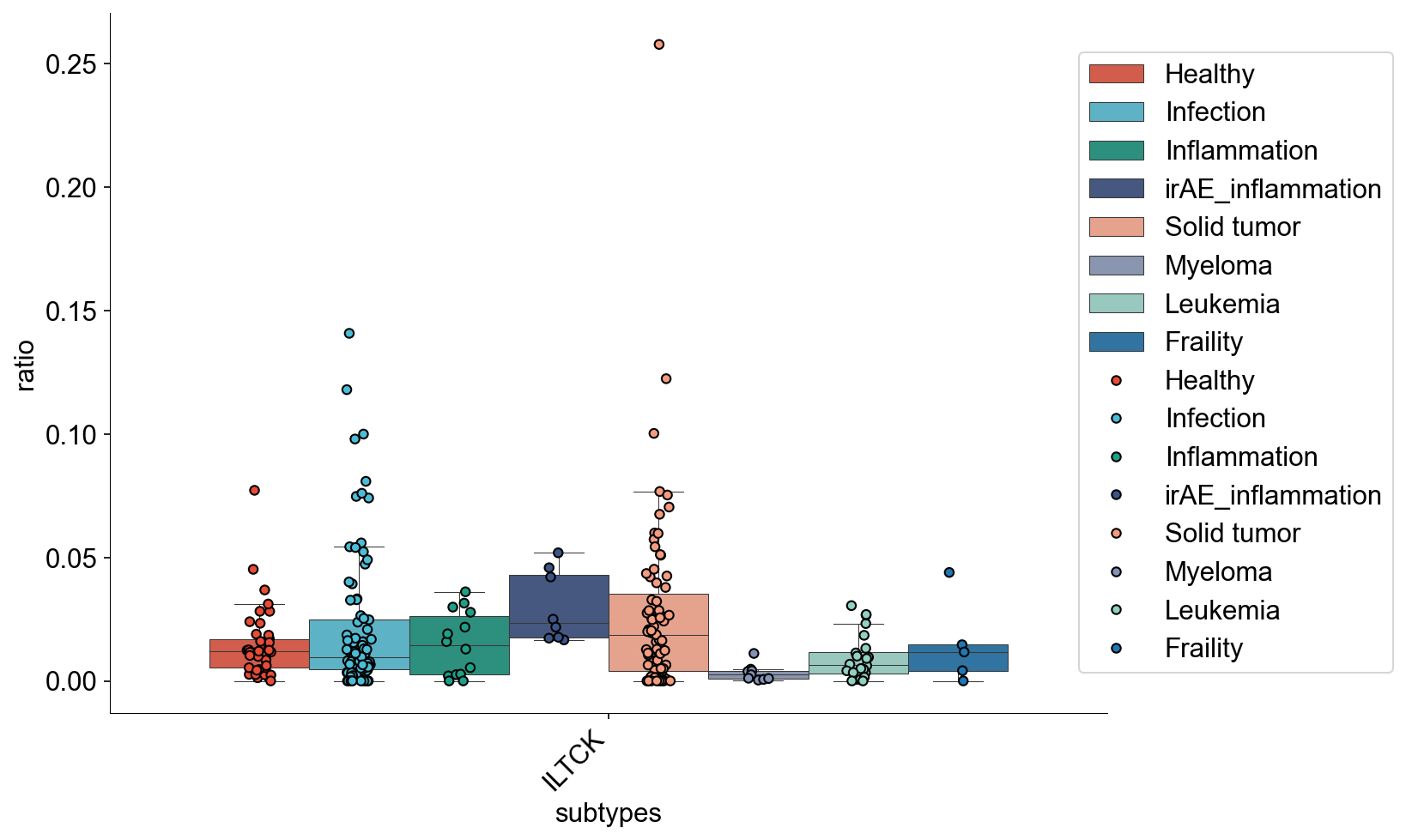
def tissue_pie_data(adata_use=adata_nogex,
groupby='disease_type', groupby_type='Healthy',
ratioby='cell_subtype_3',
ratioby_type=['ITGAE+ Trm', 'ITGB2+ Trm', 'GZMK+ Tex', 'CXCR6+ Tex'],
expanded=True):
adata = adata_use[adata_use.obs[groupby]==groupby_type]
if expanded == 'large':
adata_use = adata[
(adata.obs['expansion_level'].str.contains('100')) |
(adata.obs['expansion_level'].str.contains('50'))
]
elif expanded == True:
adata_use = adata[
adata.obs['cc_aa_identity_size']>=3
]
else:
adata_use = adata
pie_list = []
for i in ratioby_type:
TNum = pd.Series(Counter(adata_use.obs[ratioby])).loc[i]
pie_list.append(TNum)
OtherNum = len(adata_use.obs)-sum(pie_list)
pie_list.append(OtherNum)
ratio = len(adata_use.obs)/len(adata.obs)
return pie_list, ratio
PlotsFunctions¶
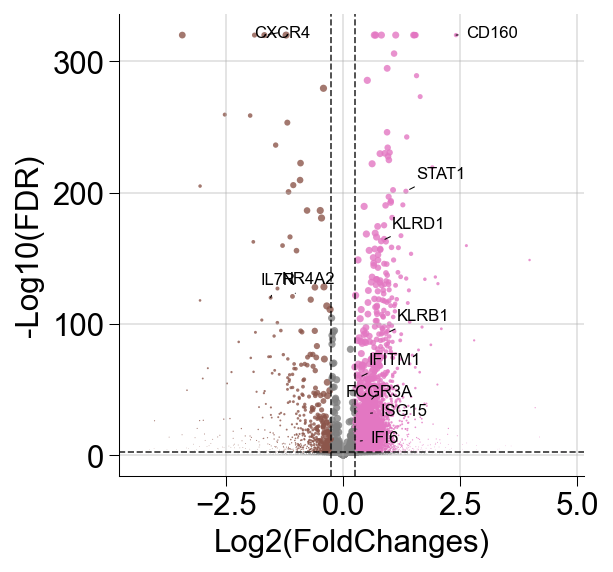
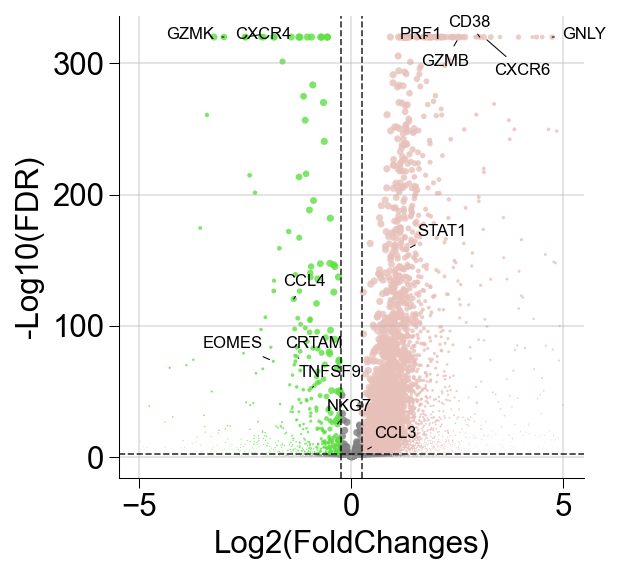
def ScanpyVolcanoPlot(
adata,
axis,
use_adjusted_p = True,
show_label=True,
label_fold_change = 2,
label_log_p = 80,
label_includes=[],
label_excludes=[],
add_grid=True,
label_size=4,
color1 = '#F09D30',
color2='#3D5FA6',
max_abs_fc=5,
dot_size=12,
size_by=None,
size_group=None,
size_reference=None
):
fig,ax = createFig()
log2foldchanges = list(map(lambda x: x[axis], adata.uns['rank_genes_groups']['logfoldchanges']))
if use_adjusted_p:
log10adjp = -np.log10(list(map(lambda x: x[axis], adata.uns['rank_genes_groups']['pvals_adj'])))
else:
log10adjp = -np.log10(list(map(lambda x: x[axis], adata.uns['rank_genes_groups']['pvals'])))
log10adjp[log10adjp==np.inf] = 320
gene_names = list(map(lambda x: x[axis], adata.uns['rank_genes_groups']['names']))
indices = list(map(lambda x: abs(x) < max_abs_fc, log2foldchanges))
size_frac = {}
if size_by is not None and size_group is not None:
_adata = adata[
adata.obs[size_by] == size_group
]
frac = (_adata[:,gene_names].X > 0).sum(0) / _adata.shape[0]
frac = np.array(frac).flatten()
if size_reference is not None:
_adata = adata[
adata.obs[size_by] == size_reference
]
frac_other = (_adata[:,gene_names].X > 0).sum(0) / _adata.shape[0]
frac_other = np.array(frac_other).flatten()
else:
_adata = adata[
adata.obs[size_by] != size_group
]
frac_other = (_adata[:,gene_names].X > 0).sum(0) / _adata.shape[0]
frac_other = np.array(frac_other).flatten()
for i in label_includes:
print(f"Fraction of gene {i} is {frac[gene_names.index(i)]}")
ax.scatter(
x = np.array(log2foldchanges)[indices],
y = np.array(log10adjp[indices]),
s = dot_size if size_by is None else [frac[indices][x[0]] * dot_size \
if x[1] > 0 \
else frac_other[indices][x[0]] * dot_size \
for x in enumerate(np.array(log2foldchanges)[indices])],
alpha=0.8,
linewidth = 0,
c = list(map(lambda x: color1 if abs(x[0]) > label_fold_change and x[1] > label_log_p and x[0] < 0 \
else color2 if abs(x[0]) > label_fold_change and x[1] > label_log_p and x[0] > 0 \
else 'gray', np.array(list(zip(log2foldchanges, log10adjp)))[indices]))
)
if add_grid:
ax.axvline(-label_fold_change, color ="black", alpha = 0.8, lw = 0.8, ls='--')
ax.axvline(label_fold_change, color ="black", alpha = 0.8, lw = 0.8, ls='--')
ax.axhline(label_log_p, color ="black", alpha = 0.8, lw = 0.8, ls='--')
ax.grid(alpha=0.4)
if show_label:
indices = list(map(lambda x: abs(x[0]) > label_fold_change and x[1] > label_log_p and abs(x[0]) < max_abs_fc, zip(log2foldchanges, log10adjp)))
all_text = []
for i,j,s in zip(np.array(log2foldchanges)[indices],np.array(log10adjp)[indices],
list(map(lambda x: x[axis], adata.uns['rank_genes_groups']['names'][indices]))):
if label_excludes is not None and label_excludes != [] and s in label_excludes:
if label_includes is not None and label_includes != [] and s in label_includes:
all_text.append(ax.annotate(
xy=(i,j),
text=s,
size=8,
xytext=(-5,5) if i < 0 else (5,5),
textcoords='offset points',
ha='center',
#va="baseline",
arrowprops=dict(
arrowstyle='-',
mutation_scale=0.005,
color='black',
lw=0.5,
ls='-',
)
))
else:
continue
from adjustText import adjust_text
adjust_text(all_text, ax=ax)
ax.set_xlabel("Log2(FoldChanges)")
ax.set_ylabel("-Log10(FDR)")
return fig,ax
def volcanoPlot(diff_exp_genes, file_name=str):
result = pd.DataFrame()
diff_exp_genes[1] = diff_exp_genes[1].astype('float')
result['x'] = diff_exp_genes[1]
diff_exp_genes[2] = diff_exp_genes[2].astype('float')
result['y'] = [-np.log10(x) for x in diff_exp_genes[2]]
result.replace([np.inf, -np.inf], 320, inplace=True)
#set threshold
x_threshold=1
y_threshold=2
#groupby up, normal, down
result['group'] = 'black'
result.loc[(result.x > x_threshold)&(result.y > y_threshold),'group'] = 'tab:red' #x=-+x_threshold直接截断
result.loc[(result.x < -x_threshold)&(result.y > y_threshold),'group'] = 'tab:blue' #x=-+x_threshold直接截断
result.loc[result.y < y_threshold,'group'] = 'dimgrey' #阈值以下点为灰色
print(result.head())
## xtick limit
xmin=-10
xmax=10
ymin=-20
ymax=340
## scatter
fig = plt.figure(figsize=(7,7)) #确定fig比例(h/w)
ax = fig.add_subplot()
ax.set(xlim=(xmin, xmax), ylim=(ymin, ymax), title='')
ax.scatter(result['x'], result['y'], s=6, c=result['group'])
## ax.set_ylabel('-Log10(Q value)',fontweight='bold')
## ax.set_xlabel('Log2 (fold change)',fontweight='bold')
ax.spines['right'].set_visible(False) #去掉右边框
ax.spines['top'].set_visible(False) #去掉上边框
## horizontal and vertical line
ax.vlines(-x_threshold, ymin, ymax, color='dimgrey',linestyle='dashed', linewidth=1) #画竖直线
ax.vlines(x_threshold, ymin, ymax, color='dimgrey',linestyle='dashed', linewidth=1) #画竖直线
ax.hlines(y_threshold, xmin, xmax, color='dimgrey',linestyle='dashed', linewidth=1) #画竖水平线
ax.set_xticks(range(xmin,xmax,2))#设置x轴刻度起点和步长
## ax.set_xticklabels(labels=ax.get_xticklabels(),rotation=45)
ax.set_yticks(range(ymin,ymax,40)) #设置y轴刻度起点和步长
fig.savefig(file_name, dpi=600)
plt.show()
## stats of clonotype sharing
def circos_edges(clone_df, subtypes, file_name, specific_subtype=None,
first_index='first', second_index='second'):
edges = pd.DataFrame([], columns=['source', 'target', 'value'])
if specific_subtype is not None:
for i in subtypes:
clonei_qualified = clone_df[(clone_df[first_index]==specific_subtype) |
(clone_df[second_index]==specific_subtype)]
clonej_qualified = clonei_qualified[(clonei_qualified[first_index]==i) |
(clonei_qualified[second_index]==i)]
rows = [specific_subtype, i, len(clonej_qualified)]
edges.loc[len(edges.index)] = rows
else:
for idx, i in enumerate(subtypes):
if idx < len(subtypes)-1:
for j in subtypes[idx+1:]:
clonei_qualified = clone_df[(clone_df[first_index]==i) | (clone_df[second_index]==i)]
clonej_qualified = clonei_qualified[(clonei_qualified[first_index]==j) |
(clonei_qualified[second_index]==j)]
rows = [i, j, len(clonej_qualified)]
edges.loc[len(edges.index)] = rows
else: pass
edges = edges[edges.ne(0).all(axis=1)]
edges.to_csv(file_name, header=True, index=False)
return edges
## Figure2D, stats of circos plot
def sanke_plot(circos_df, column1, column2, file_name):
circos_df['sg'] = None
circos_df.loc[circos_df['source']==column1,'sg'] = 0
circos_df.loc[circos_df['source']==column2,'sg'] = 2
circos_df.loc[circos_df['sg'].isna(),'sg'] = 1
circos_df['tg'] = None
circos_df.loc[circos_df['target']==column1,'tg'] = 0
circos_df.loc[circos_df['target']==column2,'tg'] = 2
circos_df.loc[circos_df['tg'].isna(),'tg'] = 1
circos_df = circos_df[(circos_df['sg']!=1) | (circos_df['tg']!=1)]
try:
inter_value = circos_df[(circos_df['sg']==0) & (circos_df['tg']==2)].value[0]
except:
inter_value = 0
circos_df = circos_df[(circos_df['sg']!=0) | (circos_df['tg']!=2)]
col1_num = sum(circos_df[(circos_df['source']==column1) |
(circos_df['target']==column1)].value)
col2_num = sum(circos_df[(circos_df['source']==column2) |
(circos_df['target']==column2)].value)
circos_df['value_raw'] = circos_df['value']
circos_df.loc[(circos_df['sg']==0) | (circos_df['tg']==0),
'value'] = (circos_df.loc[(circos_df['sg']==0) | (circos_df['tg']==0),
'value']/col1_num)*100
circos_df.loc[(circos_df['sg']==2) | (circos_df['tg']==2),
'value'] = (circos_df.loc[(circos_df['sg']==2) | (circos_df['tg']==2),
'value']/col2_num)*100
circos_df.to_csv(file_name)
venn_df = [col1_num, col2_num, inter_value]
return circos_df,venn_df
## Figure2D, stats of circos plot
def sanke_plot3(circos_df, column1, column2, column3, file_name):
circos_df['sg'] = None
circos_df.loc[circos_df['source'].isin([column1,column2,column3]),'sg'] = 0
circos_df.loc[circos_df['sg'].isna(),'sg'] = 1
circos_df['tg'] = None
circos_df.loc[circos_df['target'].isin([column1,column2,column3]),'tg'] = 0
circos_df.loc[circos_df['tg'].isna(),'tg'] = 1
circos_df = circos_df[(circos_df['sg']==0)|(circos_df['tg']==0)]
inter_value12 = int(circos_df[(circos_df['source']==column1) &
(circos_df['target']==column2)].value)
inter_value13 = int(circos_df[(circos_df['source']==column1) &
(circos_df['target']==column3)].value)
inter_value23 = int(circos_df[(circos_df['source']==column2) &
(circos_df['target']==column3)].value)
circos_df = circos_df[circos_df['tg']!=0]
col1_num = sum(circos_df[circos_df['source']==column1].value)
col2_num = sum(circos_df[circos_df['source']==column2].value)
col3_num = sum(circos_df[circos_df['source']==column3].value)
circos_df['value_raw'] = circos_df['value']
circos_df.loc[circos_df['source']==column1, 'value'] = (
circos_df.loc[circos_df['source']==column1, 'value']/col1_num
)*100
circos_df.loc[circos_df['source']==column2, 'value'] = (
circos_df.loc[circos_df['source']==column2, 'value']/col1_num
)*100
circos_df.loc[circos_df['source']==column3, 'value'] = (
circos_df.loc[circos_df['source']==column3, 'value']/col1_num
)*100
circos_df.to_csv(file_name)
venn_df = [col1_num, col2_num, inter_value12, col3_num, inter_value13, inter_value23, 0]
return circos_df,venn_df
from matplotlib.patches import PathPatch
def adjust_box_widths(g, fac):
"""
Adjust the withs of a seaborn-generated boxplot.
"""
# iterating through Axes instances
for ax in g.axes:
# iterating through axes artists:
for c in ax.get_children():
# searching for PathPatches
if isinstance(c, PathPatch):
# getting current width of box:
p = c.get_path()
verts = p.vertices
verts_sub = verts[:-1]
xmin = np.min(verts_sub[:, 0])
xmax = np.max(verts_sub[:, 0])
xmid = 0.5*(xmin+xmax)
xhalf = 0.5*(xmax - xmin)
# setting new width of box
xmin_new = xmid-fac*xhalf
xmax_new = xmid+fac*xhalf
verts_sub[verts_sub[:, 0] == xmin, 0] = xmin_new
verts_sub[verts_sub[:, 0] == xmax, 0] = xmax_new
# setting new width of median line
for l in ax.lines:
if np.all(l.get_xdata() == [xmin, xmax]):
l.set_xdata([xmin_new, xmax_new])
adata_ex = adata_nogex[adata_nogex.obs['cell_subtype_3'].str.contains('Tex')]
adata_gene.uns['log1p']['base'] = None
adata_ex_allg = adata_gene[adata_gene.obs['cell_subtype_3'].str.contains('Tex')]
MainFigure2¶
DEG Analysis¶
rt_path = '/Users/snow/Desktop/2023-NM-Revision-GEX-HuARdb-Notebooks/data/'
adata_tex = sc.read_h5ad(rt_path+'20231210Tex.retrain_vae.h5ad')
adata_tex_allg = adata_gene[adata_tex.obs.index]
## import matplotlib.patches as mpatch
from adjustText import adjust_text
adata = adata_ex_allg
palette=subtype_color
fig,ax=createFig(figsize=(10,4))
groupby='cell_subtype_3'
group_frac_dict = {}
for size_obs_group in np.unique(adata.obs[groupby]):
_adata = adata[
adata.obs[groupby] == size_obs_group
]
frac = dict(zip(_adata.var.index, np.array((_adata.X > 0).sum(0) / _adata.shape[0]).flatten()))
group_frac_dict[size_obs_group] = frac
result = []
columns = list(pd.DataFrame(adata.uns['rank_genes_groups']['logfoldchanges']).columns)
for i,j,k in zip(pd.DataFrame(adata.uns['rank_genes_groups']['logfoldchanges']).to_numpy(),
pd.DataFrame(adata.uns['rank_genes_groups']['names']).to_numpy(),
pd.DataFrame(adata.uns['rank_genes_groups']['pvals_adj']).to_numpy(),
):
for e,(m,n,p) in enumerate(zip(i,j,-np.log10(k))):
result.append((columns[e],m,n,p))
result = pd.DataFrame(result, columns=[groupby,'logfoldchanges','gene_name','pvals_adj'])
result = result.sort_values(groupby)
result['size'] = list(map(lambda x: group_frac_dict[x[0]][x[1]], zip(result['cell_subtype_3'],result['gene_name'])))
result[groupby] = result[groupby].astype('category') # make sure the column uses pandas categorical type
# make a numerical column and add some jitter
result[groupby + '_codes'] = result[groupby].cat.codes + np.random.uniform(-0.3, 0.3, len(result))
_data = result[
np.array(result['logfoldchanges'] > 0) &
np.array(np.abs(result['logfoldchanges']) < 10) &
(np.array(np.abs(result['logfoldchanges']) < 0.25) |
np.array(np.abs(result['pvals_adj']) <= 2))
]
sns.scatterplot(
data=_data,
x=groupby + '_codes',
y = 'logfoldchanges',
ax=ax,
marker='o',
size='size',
sizes=(0,12),
color='#D7D7D7',
zorder=-1,
legend=False
)
_data = result[
np.array(result['logfoldchanges'] > 0) &
np.array(np.abs(result['logfoldchanges']) < 10) &
np.array(np.abs(result['logfoldchanges']) >= 0.25) &
np.array(np.abs(result['pvals_adj']) > 2)
]
for i in np.unique(_data[groupby]):
st = sns.scatterplot(
data=_data[_data[groupby] == i],
x=groupby + '_codes',
y = 'logfoldchanges',
ax=ax,
marker='o',
size='size',
sizes=(0,12),
color=subtype_color[i],
zorder=-1,
legend=False
)
_data = result[
np.array(result['logfoldchanges'] < 0) &
np.array(np.abs(result['logfoldchanges']) < 5) &
(np.array(np.abs(result['logfoldchanges']) < 0.25) |
np.array(np.abs(result['pvals_adj']) <= 2))
]
sns.scatterplot(
data=_data,
x=groupby + '_codes',
y = 'logfoldchanges',
ax=ax,
marker='o',
size='size',
sizes=(0,12),
color='#D7D7D7',
zorder=-1,
legend=False
)
_data = result[
np.array(result['logfoldchanges'] < 0) &
np.array(np.abs(result['logfoldchanges']) < 5) &
np.array(np.abs(result['logfoldchanges']) > 0.25) &
np.array(np.abs(result['pvals_adj']) > 2)
]
sns.scatterplot(
data=_data,
x=groupby + '_codes',
y = 'logfoldchanges',
ax=ax,
marker='o',
size='size',
sizes=(0,12),
color='#D7D7D7',
zorder=-1,
legend=False
)
all_collections = list(filter(lambda x:
isinstance(x, matplotlib.collections.PathCollection),
st.get_children()
))
mdict = {}
all_text = []
for e,(i,j,k,q )in enumerate(zip(pd.DataFrame(adata.uns['rank_genes_groups']['logfoldchanges']).to_numpy().T,
pd.DataFrame(adata.uns['rank_genes_groups']['names']).to_numpy().T,
pd.DataFrame(adata.uns['rank_genes_groups']['pvals_adj']).to_numpy().T,
[
[
'CD27',
'CD74',
'CD38',
'CCL3',
'CCL4',
'CCL4L2',
'CCL5',
'GZMK',
'NKG7',
],[
'ITGAE',
'KLRD1',
'KLRC1',
'KIR2DL4',
'TNFRSF18',
'TNFRSF25',
'GNLY',
'ZEB2',
'NFKB1',
'NFKB2',
'RORA'
],[
'CD81',
'CD70',
'LCK',
'ZAP70',
'XBP1',
'IKZF1',
'RUNX1',
]
])):
for _,(m,n,p) in enumerate(zip(i,j,k)):
if n in q:
x,y=result.loc[
np.array(result[groupby].cat.codes == e) &
np.array(result['gene_name'] == n),
groupby + '_codes'
].iloc[0],m
ax.scatter(x,y,lw=.3, edgecolor='black',c=subtype_color[[
'GZMK+ Tex', 'ITGAE+ Tex', 'S100A11+ Tex'
][e]], s=group_frac_dict[[
'GZMK+ Tex', 'ITGAE+ Tex', 'S100A11+ Tex'
][e]][n] * 12)
fig.savefig('./figures/2024023_triple_tex_deg.png', dpi=600)
for e,(i,j,k,q )in enumerate(zip(pd.DataFrame(adata.uns['rank_genes_groups']['logfoldchanges']).to_numpy().T,
pd.DataFrame(adata.uns['rank_genes_groups']['names']).to_numpy().T,
pd.DataFrame(adata.uns['rank_genes_groups']['pvals_adj']).to_numpy().T,
[
[
'CD27',
'CD74',
'CD38',
'CCL3',
'CCL4',
'CCL4L2',
'CCL5',
'GZMK',
'NKG7',
],[
'ITGAE',
'KLRD1',
'KLRC1',
'KIR2DL4',
'TNFRSF18',
'TNFRSF25',
'GNLY',
'ZEB2',
'NFKB1',
'NFKB2',
'RORA'
],[
'CD81',
'CD70',
'LCK',
'ZAP70',
'XBP1',
'IKZF1',
'RUNX1',
]
])):
for _,(m,n,p) in enumerate(zip(i,j,k)):
if n in q:
x,y=result.loc[
np.array(result[groupby].cat.codes == e) &
np.array(result['gene_name'] == n),
groupby + '_codes'
].iloc[0],m
all_text.append(
ax.annotate(
xy=(x,y),
text=n,
size=8,
xytext=(1,40),
textcoords='offset points',
ha='center',
#va="baseline",
arrowprops=dict(
arrowstyle='-',
mutation_scale=0.005,
color='black',
lw=0.5,
ls='-',
)
)
)
# adjust_text(all_text,ax=ax)
plt.show()
fig.savefig('figures/2024023_triple_tex_deg.pdf')
/var/folders/lw/6xch0fpj02j61gwkgwfbv2940000gn/T/ipykernel_43451/1168359865.py:15: RuntimeWarning: divide by zero encountered in log10
for e,(m,n,p) in enumerate(zip(i,j,-np.log10(k))):
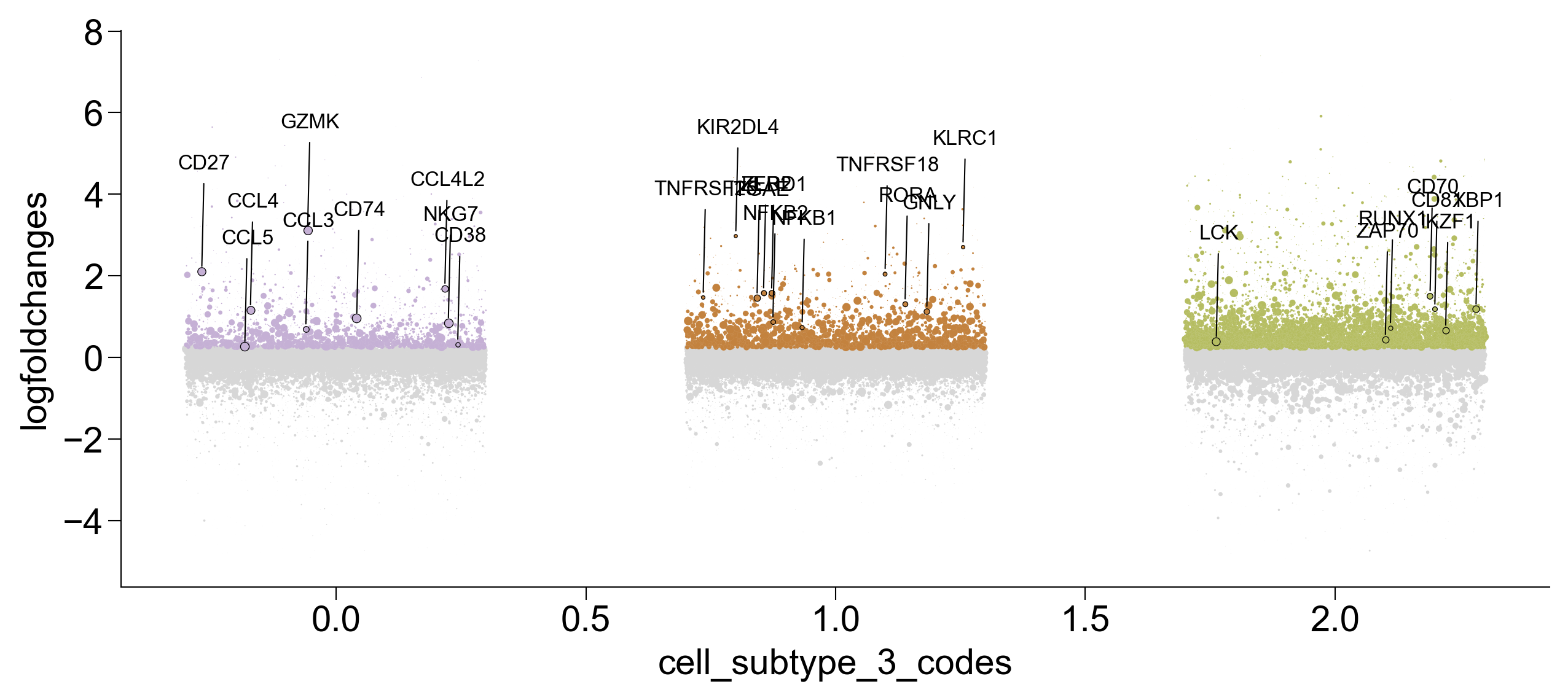
GO Term Analysis¶
## deg_tex,deg_tex_up,deg_tex_dw,enr_tex_up,enr_tex_dw
gzmk_ls = DEG_analysis(adata_ex_allg, groupby='cell_subtype_3',
query_subtype='GZMK+ Tex', reference_subtype='rest', dw=False)
## deg_tex,deg_tex_up,deg_tex_dw,enr_tex_up,enr_tex_dw
itgae_ls = DEG_analysis(adata_ex_allg, groupby='cell_subtype_3',
query_subtype='ITGAE+ Tex', reference_subtype='rest', dw=False)
## deg_tex,deg_tex_up,deg_tex_dw,enr_tex_up,enr_tex_dw
xbp_ls = DEG_analysis(adata_ex_allg, groupby='cell_subtype_3',
query_subtype='XBP1+ Tex', reference_subtype='rest', dw=False)
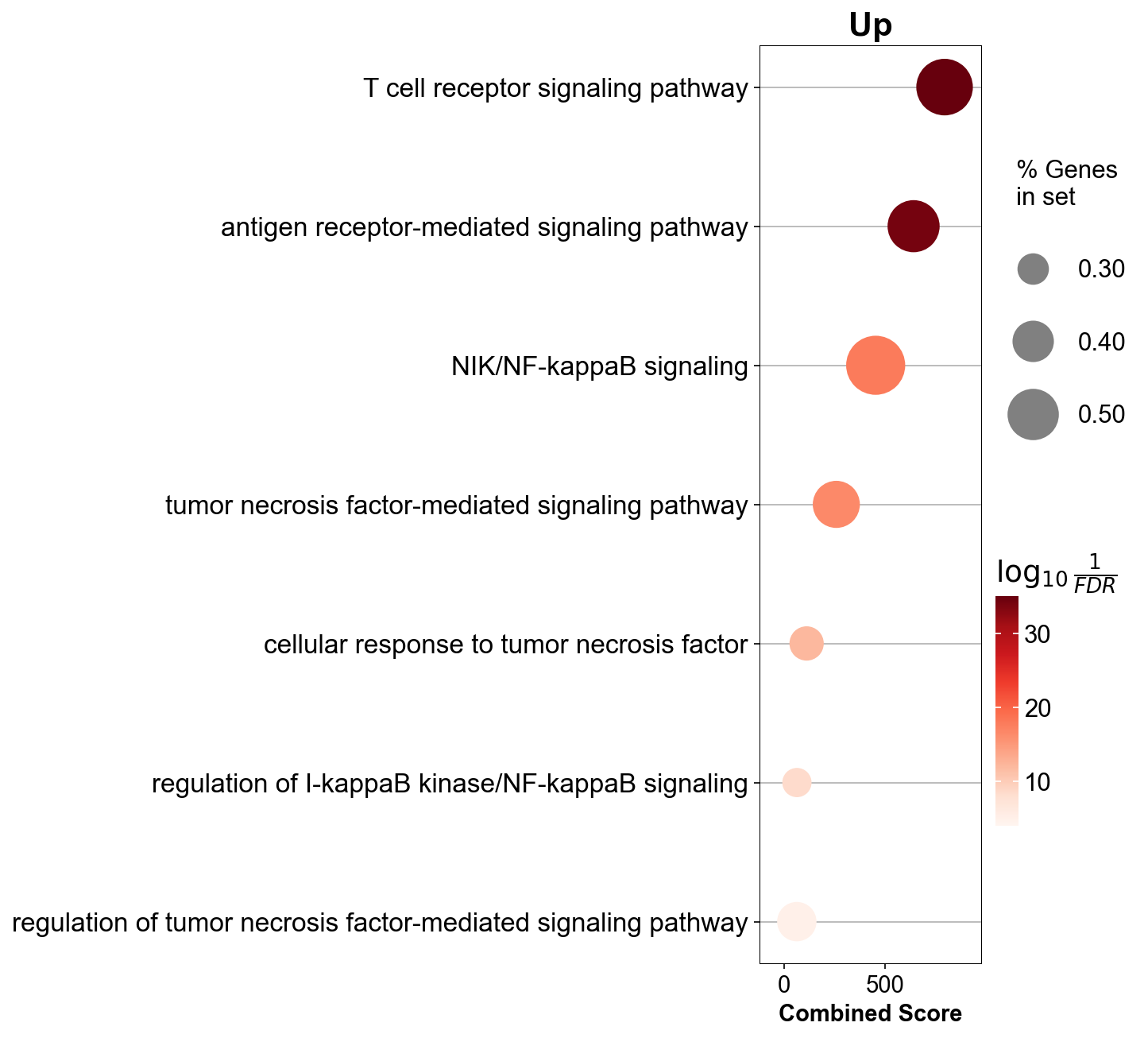
gzmk_go = gzmk_ls[3].res2d[gzmk_ls[3].res2d['Adjusted P-value']<0.05]
itgae_go = itgae_ls[3].res2d[itgae_ls[3].res2d['Adjusted P-value']<0.05]
xbp_go = xbp_ls[3].res2d[xbp_ls[3].res2d['Adjusted P-value']<0.05]
gzmk_go_inter = gzmk_go.append([itgae_go,xbp_go])
gzmk_go_inter = gzmk_go_inter.drop_duplicates(['Term'], keep=False)
gzmk_go_inter[0:50]
| Gene_set | Term | Overlap | P-value | Adjusted P-value | Old P-value | Old Adjusted P-value | Odds Ratio | Combined Score | Genes | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | GO_Biological_Process_2021 | cellular response to interferon-gamma (GO:0071... | 24/121 | 4.791630e-23 | 9.257430e-20 | 0 | 0 | 23.513522 | 1208.420691 | HLA-DRB5;VCAM1;CCL3L1;STAT1;HLA-C;HLA-A;HLA-F;... |
| 1 | GO_Biological_Process_2021 | interferon-gamma-mediated signaling pathway (G... | 18/68 | 5.232690e-20 | 5.054779e-17 | 0 | 0 | 33.327887 | 1479.650758 | HLA-DRB5;VCAM1;STAT1;HLA-C;HLA-A;HLA-F;OASL;HL... |
| 11 | GO_Biological_Process_2021 | antigen processing and presentation of exogeno... | 9/73 | 1.599254e-07 | 2.574799e-05 | 0 | 0 | 12.482052 | 195.326127 | PSMB3;PSME1;HLA-C;PSME2;HLA-A;HLA-F;PSMB10;HLA... |
| 12 | GO_Biological_Process_2021 | antigen processing and presentation of exogeno... | 9/78 | 2.857599e-07 | 4.246831e-05 | 0 | 0 | 11.574618 | 174.407666 | PSMB3;PSME1;HLA-C;PSME2;HLA-A;HLA-F;PSMB10;HLA... |
| 14 | GO_Biological_Process_2021 | antigen processing and presentation of endogen... | 5/14 | 3.619638e-07 | 4.662094e-05 | 0 | 0 | 48.574238 | 720.439570 | HLA-DRA;HLA-A;HLA-F;HLA-DRB1;HLA-E |
| 15 | GO_Biological_Process_2021 | antigen processing and presentation of endogen... | 4/7 | 5.905587e-07 | 6.711526e-05 | 0 | 0 | 116.099853 | 1665.126939 | HLA-C;HLA-A;HLA-F;HLA-E |
| 16 | GO_Biological_Process_2021 | antigen processing and presentation of endogen... | 4/7 | 5.905587e-07 | 6.711526e-05 | 0 | 0 | 116.099853 | 1665.126939 | HLA-C;HLA-A;HLA-F;HLA-E |
| 17 | GO_Biological_Process_2021 | antigen processing and presentation of exogeno... | 4/8 | 1.170412e-06 | 1.256242e-04 | 0 | 0 | 87.070485 | 1189.222132 | HLA-C;HLA-A;HLA-F;HLA-E |
| 18 | GO_Biological_Process_2021 | cellular response to interleukin-1 (GO:0071347) | 11/155 | 1.934576e-06 | 1.967158e-04 | 0 | 0 | 6.814236 | 89.645517 | CCL3L1;UBB;PSMB3;CCL5;CCL4;PSME1;CCL3;PSME2;RB... |
| 21 | GO_Biological_Process_2021 | cellular response to tumor necrosis factor (GO... | 12/194 | 2.797056e-06 | 2.456323e-04 | 0 | 0 | 5.897034 | 75.405045 | CCL3L1;PSMB3;STAT1;TNFRSF9;CCL5;CCL4;PSME1;CCL... |
| 23 | GO_Biological_Process_2021 | positive regulation of leukocyte mediated cyto... | 6/43 | 9.487500e-06 | 7.637438e-04 | 0 | 0 | 14.221261 | 164.476500 | CRTAM;HLA-DRA;HLA-A;HLA-F;HLA-DRB1;HLA-E |
| 24 | GO_Biological_Process_2021 | positive regulation of T cell mediated cytotox... | 5/26 | 1.062088e-05 | 8.207812e-04 | 0 | 0 | 20.804888 | 238.271919 | HLA-DRA;HLA-A;HLA-F;HLA-DRB1;HLA-E |
| 25 | GO_Biological_Process_2021 | negative regulation by host of viral transcrip... | 4/13 | 1.142322e-05 | 8.488327e-04 | 0 | 0 | 38.688204 | 440.266452 | HDAC1;CCL5;CCL4;CCL3 |
| 27 | GO_Biological_Process_2021 | positive regulation of CD4-positive, CD25-posi... | 3/5 | 1.495090e-05 | 9.960390e-04 | 0 | 0 | 130.046053 | 1444.907793 | IFNG;HLA-DRA;HLA-DRB1 |
| 28 | GO_Biological_Process_2021 | positive regulation of natural killer cell che... | 3/5 | 1.495090e-05 | 9.960390e-04 | 0 | 0 | 130.046053 | 1444.907793 | CCL5;CCL4;CCL3 |
| 30 | GO_Biological_Process_2021 | regulation of T cell mediated cytotoxicity (GO... | 5/29 | 1.864039e-05 | 1.161717e-03 | 0 | 0 | 18.201512 | 198.217741 | HLA-DRA;HLA-A;HLA-F;HLA-DRB1;HLA-E |
| 32 | GO_Biological_Process_2021 | regulation of lymphocyte activation (GO:0051249) | 4/15 | 2.141506e-05 | 1.216880e-03 | 0 | 0 | 31.650781 | 340.290711 | SIT1;CCL5;CRTAM;IKZF3 |
| 33 | GO_Biological_Process_2021 | negative regulation of single stranded viral R... | 4/15 | 2.141506e-05 | 1.216880e-03 | 0 | 0 | 31.650781 | 340.290711 | APOBEC3C;APOBEC3G;MPHOSPH8;RESF1 |
| 36 | GO_Biological_Process_2021 | T cell mediated immunity (GO:0002456) | 4/16 | 2.829516e-05 | 1.448035e-03 | 0 | 0 | 29.011747 | 303.834800 | CD8A;CRTAM;BTN3A2;HLA-A |
| 37 | GO_Biological_Process_2021 | peptide antigen assembly with MHC protein comp... | 3/6 | 2.964657e-05 | 1.448035e-03 | 0 | 0 | 86.692982 | 903.875260 | HLA-DMA;HLA-DRA;HLA-DRB1 |
| 38 | GO_Biological_Process_2021 | positive regulation of cell-cell adhesion medi... | 3/6 | 2.964657e-05 | 1.448035e-03 | 0 | 0 | 86.692982 | 903.875260 | CCL5;CD3E;CXCL13 |
| 39 | GO_Biological_Process_2021 | neutrophil migration (GO:1990266) | 7/77 | 3.030208e-05 | 1.448035e-03 | 0 | 0 | 8.794196 | 91.497406 | CCL3L1;CCL5;ITGB2;CCL4;PECAM1;CCL3;CXCL13 |
| 40 | GO_Biological_Process_2021 | modulation by host of symbiont process (GO:005... | 5/32 | 3.072953e-05 | 1.448035e-03 | 0 | 0 | 16.176663 | 168.080169 | HDAC1;CCL5;CCL4;CCL3;NUCKS1 |
| 41 | GO_Biological_Process_2021 | antigen processing and presentation of peptide... | 5/33 | 3.587804e-05 | 1.650390e-03 | 0 | 0 | 15.598135 | 159.652921 | BCAP31;HLA-C;HLA-A;HLA-F;HLA-E |
| 42 | GO_Biological_Process_2021 | regulation of single stranded viral RNA replic... | 4/18 | 4.671692e-05 | 2.051298e-03 | 0 | 0 | 24.864695 | 247.935919 | APOBEC3C;APOBEC3G;MPHOSPH8;RESF1 |
| 43 | GO_Biological_Process_2021 | positive regulation of lymphocyte chemotaxis (... | 4/18 | 4.671692e-05 | 2.051298e-03 | 0 | 0 | 24.864695 | 247.935919 | CCL5;CCL4;CCL3;CXCL13 |
| 44 | GO_Biological_Process_2021 | regulation of natural killer cell chemotaxis (... | 3/7 | 5.143883e-05 | 2.208440e-03 | 0 | 0 | 65.016447 | 642.045042 | CCL5;CCL4;CCL3 |
| 45 | GO_Biological_Process_2021 | anaphase-promoting complex-dependent catabolic... | 7/84 | 5.330332e-05 | 2.238740e-03 | 0 | 0 | 7.991883 | 78.636229 | ANAPC16;UBB;PSMB3;PSME1;PSME2;PSMB10;PSMB9 |
| 46 | GO_Biological_Process_2021 | positive regulation of T cell mediated immunit... | 5/36 | 5.540566e-05 | 2.277526e-03 | 0 | 0 | 14.086497 | 138.059348 | HLA-DRA;HLA-A;HLA-F;HLA-DRB1;HLA-E |
| 47 | GO_Biological_Process_2021 | tumor necrosis factor-mediated signaling pathw... | 8/116 | 6.107338e-05 | 2.396148e-03 | 0 | 0 | 6.530809 | 63.371276 | PSMB3;STAT1;TNFRSF9;PSME1;PSME2;CD27;PSMB10;PSMB9 |
| 48 | GO_Biological_Process_2021 | regulation of interferon-gamma production (GO:... | 7/86 | 6.201212e-05 | 2.396148e-03 | 0 | 0 | 7.788766 | 75.458971 | CRTAM;HLA-DPB1;BTN3A2;HLA-A;HLA-DRB1;HAVCR2;HL... |
| 49 | GO_Biological_Process_2021 | regulation of neuron death (GO:1901214) | 7/86 | 6.201212e-05 | 2.396148e-03 | 0 | 0 | 7.788766 | 75.458971 | CD200R1;IFNG;UBB;CCL5;ITGB2;PARK7;HLA-F |
| 50 | GO_Biological_Process_2021 | positive regulation of alpha-beta T cell activ... | 4/20 | 7.263800e-05 | 2.751698e-03 | 0 | 0 | 21.754405 | 207.319970 | HLA-DRA;HLA-A;HLA-DRB1;HLA-E |
| 51 | GO_Biological_Process_2021 | regulation of cysteine-type endopeptidase acti... | 7/89 | 7.722786e-05 | 2.869312e-03 | 0 | 0 | 7.502668 | 71.040887 | BCAP31;ARL6IP1;F2R;CASP1;IFI6;PMAIP1;CYCS |
| 52 | GO_Biological_Process_2021 | lymphocyte migration (GO:0072676) | 5/40 | 9.314144e-05 | 3.395269e-03 | 0 | 0 | 12.474083 | 115.776851 | CCL3L1;CCL5;CCL4;CRTAM;CCL3 |
| 53 | GO_Biological_Process_2021 | pre-replicative complex assembly (GO:0036388) | 6/64 | 9.534653e-05 | 3.411287e-03 | 0 | 0 | 9.062529 | 83.900824 | UBB;PSMB3;PSME1;PSME2;PSMB10;PSMB9 |
| 55 | GO_Biological_Process_2021 | regulation of CD8-positive, alpha-beta T cell ... | 3/9 | 1.213568e-04 | 4.186810e-03 | 0 | 0 | 43.339912 | 390.786254 | CRTAM;HLA-A;HLA-E |
| 56 | GO_Biological_Process_2021 | lymphocyte chemotaxis (GO:0048247) | 5/44 | 1.480644e-04 | 4.979299e-03 | 0 | 0 | 11.192421 | 98.693238 | CCL3L1;CCL5;CCL4;CCL3;CXCL13 |
| 59 | GO_Biological_Process_2021 | neutrophil chemotaxis (GO:0030593) | 6/70 | 1.574189e-04 | 5.045310e-03 | 0 | 0 | 8.210417 | 71.895336 | CCL3L1;CCL5;ITGB2;CCL4;CCL3;CXCL13 |
| 62 | GO_Biological_Process_2021 | positive regulation of I-kappaB kinase/NF-kapp... | 9/171 | 1.725009e-04 | 5.290027e-03 | 0 | 0 | 4.906657 | 42.516711 | CD74;NDFIP1;F2R;CASP1;FASLG;RBCK1;CFLAR;TRIM22... |
| 63 | GO_Biological_Process_2021 | regulation of proteolysis involved in cellular... | 4/25 | 1.812497e-04 | 5.471476e-03 | 0 | 0 | 16.570589 | 142.766146 | ATP5IF1;UBB;PSME1;PSME2 |
| 64 | GO_Biological_Process_2021 | granulocyte chemotaxis (GO:0071621) | 6/73 | 1.985882e-04 | 5.902654e-03 | 0 | 0 | 7.841592 | 66.843902 | CCL3L1;CCL5;ITGB2;CCL4;CCL3;CXCL13 |
| 65 | GO_Biological_Process_2021 | regulation of cytokine-mediated signaling path... | 6/74 | 2.140505e-04 | 6.265843e-03 | 0 | 0 | 7.725882 | 65.278286 | CD74;IFNG;STAT1;PTPN6;RBCK1;CFLAR |
| 66 | GO_Biological_Process_2021 | regulation of transcription from RNA polymeras... | 6/75 | 2.304460e-04 | 6.645100e-03 | 0 | 0 | 7.613527 | 63.767046 | UBB;PSMB3;PSME1;PSME2;PSMB10;PSMB9 |
| 67 | GO_Biological_Process_2021 | positive regulation of cellular respiration (G... | 3/11 | 2.343354e-04 | 6.657882e-03 | 0 | 0 | 32.501645 | 271.673355 | IFNG;PARK7;ISCU |
| 68 | GO_Biological_Process_2021 | regulation of T cell proliferation (GO:0042129) | 6/76 | 2.478142e-04 | 6.938799e-03 | 0 | 0 | 7.504381 | 62.307608 | VCAM1;CCL5;HLA-DPB1;CD3E;HLA-DRB1;HLA-DPA1 |
| 70 | GO_Biological_Process_2021 | regulation of cellular amine metabolic process... | 5/51 | 2.999415e-04 | 8.087188e-03 | 0 | 0 | 9.485860 | 76.948566 | PSMB3;PSME1;PSME2;PSMB10;PSMB9 |
| 71 | GO_Biological_Process_2021 | regulation of mRNA stability (GO:0043488) | 8/146 | 3.013859e-04 | 8.087188e-03 | 0 | 0 | 5.103269 | 41.372810 | CARHSP1;UBB;PSMB3;CIRBP;PSME1;PSME2;PSMB10;PSMB9 |
| 72 | GO_Biological_Process_2021 | positive regulation of hydrolase activity (GO:... | 8/149 | 3.457169e-04 | 9.149657e-03 | 0 | 0 | 4.993926 | 39.801039 | CCL3L1;RGS1;CCL5;F2R;CCL4;CCL3;CALM3;MAPRE2 |
| 73 | GO_Biological_Process_2021 | regulation of cellular amino acid metabolic pr... | 5/54 | 3.926326e-04 | 1.025089e-02 | 0 | 0 | 8.903738 | 69.828782 | PSMB3;PSME1;PSME2;PSMB10;PSMB9 |
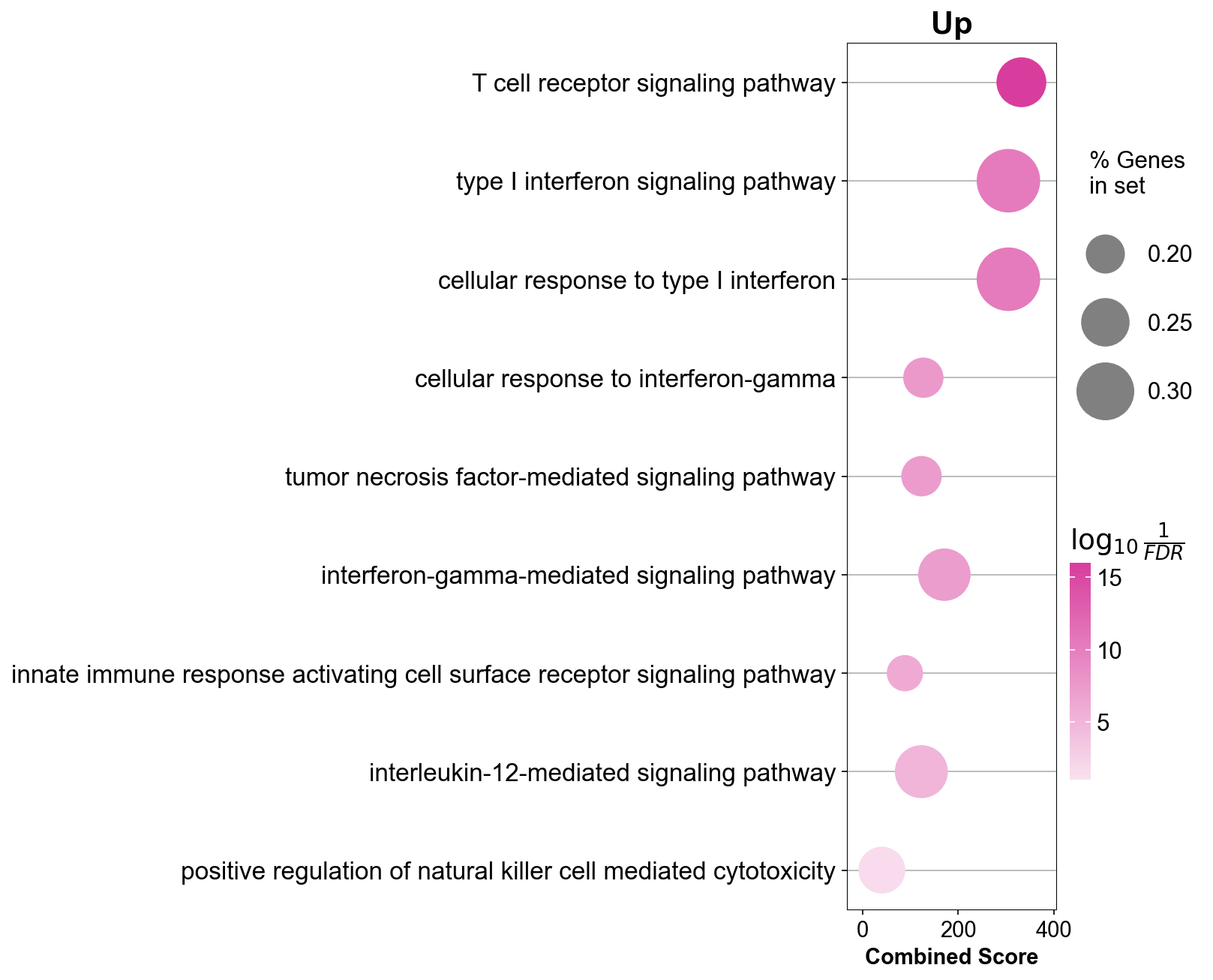
gzmk_ls[3].res2d.Term = gzmk_ls[3].res2d.Term.str.split(" \(GO").str[0]
## dotplot
gseapy.dotplot(gzmk_ls[3].res2d.iloc[[0,18,21,52],:], size=20,
cmap=make_colormap(['#c8bad8', '#5f0caf']),
figsize=(3,5), vmin=1.3)
plt.savefig('figures/gzmk_go_up.pdf')
plt.show()
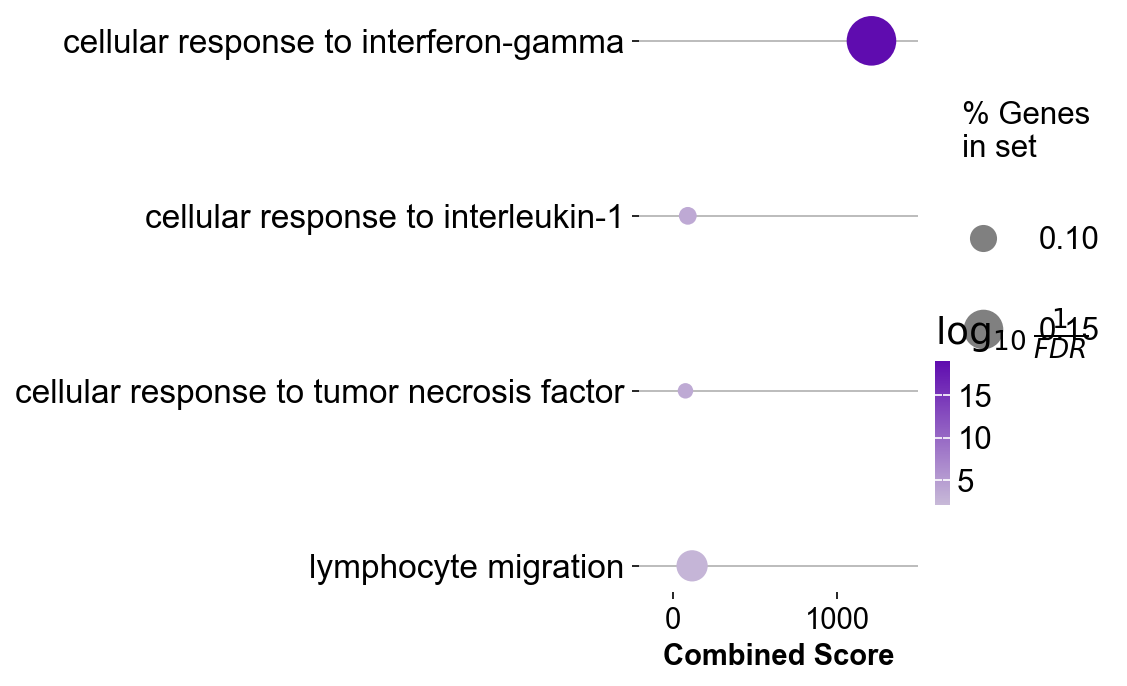
itgae_go_inter = itgae_go.append([gzmk_go,xbp_go])
itgae_go_inter = itgae_go_inter.drop_duplicates(['Term'], keep=False)
itgae_go_inter[0:50]
| Gene_set | Term | Overlap | P-value | Adjusted P-value | Old P-value | Old Adjusted P-value | Odds Ratio | Combined Score | Genes | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | GO_Biological_Process_2021 | cytoplasmic translation (GO:0002181) | 23/93 | 4.144822e-19 | 1.107496e-15 | 0 | 0 | 17.113752 | 724.378194 | EIF4A2;EIF4A1;RPL32;RPLP1;RPL22;RPL36A;GSPT1;R... |
| 1 | GO_Biological_Process_2021 | nuclear-transcribed mRNA catabolic process, no... | 24/113 | 3.216675e-18 | 4.297477e-15 | 0 | 0 | 14.069338 | 566.687397 | RPL32;RBM8A;RPLP1;EIF4A3;RPL22;RPL36A;GSPT1;RP... |
| 3 | GO_Biological_Process_2021 | nuclear-transcribed mRNA catabolic process (GO... | 25/171 | 6.698644e-15 | 4.474694e-12 | 0 | 0 | 8.931654 | 291.501234 | RPL32;RBM8A;RPLP1;EIF4A3;RPL36A;GSPT1;PPP2CA;R... |
| 4 | GO_Biological_Process_2021 | SRP-dependent cotranslational protein targetin... | 18/90 | 1.672122e-13 | 8.935821e-11 | 0 | 0 | 12.848684 | 378.002027 | RPL32;RPLP1;RPL22;RPL36A;RPS26;RPS17;RPS27;RPS... |
| 5 | GO_Biological_Process_2021 | cotranslational protein targeting to membrane ... | 18/94 | 3.695914e-13 | 1.645914e-10 | 0 | 0 | 12.169945 | 348.381437 | RPL32;RPLP1;RPL22;RPL36A;RPS26;RPS17;RPS27;RPS... |
| 8 | GO_Biological_Process_2021 | protein targeting to ER (GO:0045047) | 18/103 | 1.913071e-12 | 5.679696e-10 | 0 | 0 | 10.876347 | 293.468972 | RPL32;RPLP1;RPL22;RPL36A;RPS26;RPS17;RPS27;RPS... |
| 10 | GO_Biological_Process_2021 | cellular macromolecule biosynthetic process (G... | 27/314 | 2.019559e-10 | 4.905692e-08 | 0 | 0 | 4.897818 | 109.333860 | RPL32;MRPL18;RPLP1;RPL36A;GSPT1;RPS17;POLR2A;R... |
| 15 | GO_Biological_Process_2021 | mRNA processing (GO:0006397) | 22/300 | 1.700742e-07 | 2.840239e-05 | 0 | 0 | 4.067121 | 63.394338 | RBM17;RBM8A;CCNH;RNMT;BUD31;EIF4A3;SRRT;YBX1;H... |
| 19 | GO_Biological_Process_2021 | rRNA processing (GO:0006364) | 16/173 | 3.921314e-07 | 5.238876e-05 | 0 | 0 | 5.187581 | 76.525481 | RPL32;RIOK3;RPLP1;RPL22;RPL36A;RPS17;RPS27;RPL... |
| 23 | GO_Biological_Process_2021 | mRNA splicing, via spliceosome (GO:0000398) | 20/274 | 6.686261e-07 | 7.444037e-05 | 0 | 0 | 4.030330 | 57.303390 | RBM17;RBM8A;BUD31;EIF4A3;SRRT;YBX1;HNRNPL;PTBP... |
| 24 | GO_Biological_Process_2021 | RNA splicing, via transesterification reaction... | 19/251 | 7.355988e-07 | 7.862080e-05 | 0 | 0 | 4.185584 | 59.111244 | RBM17;RBM8A;BUD31;EIF4A3;SRRT;YBX1;HNRNPL;PTBP... |
| 25 | GO_Biological_Process_2021 | rRNA metabolic process (GO:0016072) | 15/162 | 8.919462e-07 | 9.166462e-05 | 0 | 0 | 5.183300 | 72.202650 | RPL32;RPLP1;RPL22;RPL36A;RPS17;RPS27;RPL37A;RP... |
| 27 | GO_Biological_Process_2021 | negative regulation of gene expression (GO:001... | 21/322 | 2.175059e-06 | 2.075628e-04 | 0 | 0 | 3.571834 | 46.571195 | PTGER4;SRGN;TGFB1;CITED2;EIF4A3;GZMB;PTPN22;MI... |
| 28 | GO_Biological_Process_2021 | ncRNA processing (GO:0034470) | 16/201 | 2.888248e-06 | 2.661172e-04 | 0 | 0 | 4.396095 | 56.071573 | RPL32;RPLP1;RPL22;SRRT;RPL36A;RPS17;RPS27;RPL3... |
| 30 | GO_Biological_Process_2021 | ribosome biogenesis (GO:0042254) | 15/192 | 7.395858e-06 | 6.374752e-04 | 0 | 0 | 4.298137 | 50.780728 | RPL32;RPLP1;RPL22;RPL36A;RPS17;RPS27;RPL37A;RP... |
| 32 | GO_Biological_Process_2021 | regulation of cytokine production (GO:0001817) | 13/150 | 9.854661e-06 | 7.979289e-04 | 0 | 0 | 4.797516 | 55.303687 | SRGN;PTGER4;ITK;ANXA1;ISG15;MIF;TNFRSF1B;BATF;... |
| 33 | GO_Biological_Process_2021 | regulation of mRNA splicing, via spliceosome (... | 10/90 | 1.207072e-05 | 9.486168e-04 | 0 | 0 | 6.289304 | 71.224656 | HNRNPL;PTBP1;RBM4;RBM39;RBM8A;WTAP;NUP98;PRDX6... |
| 35 | GO_Biological_Process_2021 | RNA metabolic process (GO:0016070) | 12/133 | 1.438041e-05 | 1.059935e-03 | 0 | 0 | 5.005181 | 55.805989 | HNRNPL;ISG20;PTBP1;RBM4;RBM39;HNRNPUL1;DDX24;P... |
| 36 | GO_Biological_Process_2021 | RNA processing (GO:0006396) | 14/179 | 1.467725e-05 | 1.059935e-03 | 0 | 0 | 4.294792 | 47.797647 | IVNS1ABP;RBM39;RBM8A;HNRNPL;PPP2CA;PTBP1;RBM4;... |
| 39 | GO_Biological_Process_2021 | positive regulation of transcription, DNA-temp... | 45/1183 | 2.349261e-05 | 1.544045e-03 | 0 | 0 | 2.068337 | 22.046043 | ARF4;RNF10;TMF1;CITED2;HMGB2;RORA;YBX1;RBPJ;NR... |
| 40 | GO_Biological_Process_2021 | TRIF-dependent toll-like receptor signaling pa... | 6/30 | 2.369231e-05 | 1.544045e-03 | 0 | 0 | 12.485969 | 132.980068 | UBB;UBE2D3;IRF7;TANK;BIRC2;BIRC3 |
| 41 | GO_Biological_Process_2021 | cellular response to copper ion (GO:0071280) | 5/19 | 2.811179e-05 | 1.746854e-03 | 0 | 0 | 17.800800 | 186.540302 | PRNP;MT2A;MT1F;MT1X;MT1E |
| 42 | GO_Biological_Process_2021 | negative regulation of T cell receptor signali... | 5/19 | 2.811179e-05 | 1.746854e-03 | 0 | 0 | 17.800800 | 186.540302 | PRNP;LGALS3;PTPN22;SLA2;EZR |
| 43 | GO_Biological_Process_2021 | MyD88-independent toll-like receptor signaling... | 6/31 | 2.888869e-05 | 1.754331e-03 | 0 | 0 | 11.985918 | 125.277543 | UBB;UBE2D3;IRF7;TANK;BIRC2;BIRC3 |
| 45 | GO_Biological_Process_2021 | positive regulation of gene expression (GO:001... | 24/482 | 4.003948e-05 | 2.325771e-03 | 0 | 0 | 2.682297 | 27.159985 | PTGER4;ITK;TGFB1;CITED2;EIF4A3;DDX21;SERPINB9;... |
| 46 | GO_Biological_Process_2021 | response to sterol (GO:0036314) | 4/11 | 4.563081e-05 | 2.594160e-03 | 0 | 0 | 28.419144 | 284.047287 | TGFB1;INSIG1;RORA;TGFBR2 |
| 47 | GO_Biological_Process_2021 | positive regulation of transcription by RNA po... | 36/908 | 7.038646e-05 | 3.918180e-03 | 0 | 0 | 2.136069 | 20.424042 | ARF4;RNF10;TMF1;CITED2;HMGB2;RORA;YBX1;RBPJ;NR... |
| 48 | GO_Biological_Process_2021 | positive regulation of transcription from RNA ... | 3/5 | 7.592191e-05 | 4.071335e-03 | 0 | 0 | 74.430380 | 706.032084 | RBPJ;HIF1A;NFE2L2 |
| 49 | GO_Biological_Process_2021 | response to copper ion (GO:0046688) | 5/23 | 7.618517e-05 | 4.071335e-03 | 0 | 0 | 13.842239 | 131.256871 | PRNP;MT2A;MT1F;MT1X;MT1E |
| 50 | GO_Biological_Process_2021 | regulation of cell cycle (GO:0051726) | 17/296 | 9.894100e-05 | 5.183732e-03 | 0 | 0 | 3.090255 | 28.495199 | TSPYL2;CDKN1B;TGFB1;GADD45B;CITED2;MIF;RUNX3;S... |
| 51 | GO_Biological_Process_2021 | regulation of T cell differentiation (GO:0045580) | 6/39 | 1.119261e-04 | 5.751280e-03 | 0 | 0 | 9.076531 | 82.575295 | IFNG;CAMK4;ZBTB1;CTLA4;PRDM1;SOD1 |
| 52 | GO_Biological_Process_2021 | negative regulation of antigen receptor-mediat... | 5/25 | 1.164166e-04 | 5.869155e-03 | 0 | 0 | 12.456743 | 112.837353 | LGALS3;PRNP;PTPN22;SLA2;EZR |
| 53 | GO_Biological_Process_2021 | regulation of RNA splicing (GO:0043484) | 8/76 | 1.326985e-04 | 6.566120e-03 | 0 | 0 | 5.892609 | 52.605861 | CLK1;HNRNPL;RPS26;PTBP1;RBM39;POLR2A;JMJD6;SF1 |
| 55 | GO_Biological_Process_2021 | positive regulation of type I interferon produ... | 8/77 | 1.455511e-04 | 6.944865e-03 | 0 | 0 | 5.806912 | 51.303972 | RIOK3;DHX36;IRF7;PTPN22;ISG15;GAPDH;HSPD1;NFKB2 |
| 56 | GO_Biological_Process_2021 | negative regulation of intracellular signal tr... | 13/198 | 1.764191e-04 | 8.270030e-03 | 0 | 0 | 3.543994 | 30.629497 | MAP2K3;UBE2B;RIOK3;PDE4D;PDE3B;RORA;PPP2R5C;SL... |
| 59 | GO_Biological_Process_2021 | apoptotic process (GO:0006915) | 14/231 | 2.317475e-04 | 1.032049e-02 | 0 | 0 | 3.256888 | 27.259707 | MX1;IFI6;CXCR4;GZMB;TGFBR2;HSPD1;PPP2CA;PLSCR1... |
| 60 | GO_Biological_Process_2021 | regulation of gene expression (GO:0010468) | 39/1079 | 2.421804e-04 | 1.033753e-02 | 0 | 0 | 1.938928 | 16.143177 | ZNF331;CITED2;SRRT;CREM;JMJD1C;RORA;PTPN22;PRD... |
| 62 | GO_Biological_Process_2021 | response to zinc ion (GO:0010043) | 5/29 | 2.437367e-04 | 1.033753e-02 | 0 | 0 | 10.378499 | 86.343110 | MT2A;MT1F;MT1X;CRIP1;MT1E |
| 63 | GO_Biological_Process_2021 | transcription by RNA polymerase II (GO:0006366) | 17/320 | 2.504733e-04 | 1.045726e-02 | 0 | 0 | 2.841948 | 23.565882 | SLBP;CCNH;RNMT;NR1H2;GTF2B;SRRT;ZBTB1;DDX21;RO... |
| 65 | GO_Biological_Process_2021 | regulation of innate immune response (GO:0045088) | 7/64 | 2.743734e-04 | 1.095018e-02 | 0 | 0 | 6.138780 | 50.344264 | AKIRIN2;PTPN1;PLSCR1;RIOK3;PTPN22;BIRC2;BIRC3 |
| 66 | GO_Biological_Process_2021 | negative regulation of cellular macromolecule ... | 24/547 | 2.745742e-04 | 1.095018e-02 | 0 | 0 | 2.340958 | 19.196529 | TSPYL2;CDKN1B;TGFB1;CITED2;NR1H2;EIF4A3;HMGA1;... |
| 67 | GO_Biological_Process_2021 | postreplication repair (GO:0006301) | 6/46 | 2.858004e-04 | 1.123027e-02 | 0 | 0 | 7.485459 | 61.082971 | UBB;UBE2B;ZBTB1;ISG15;UBE2A;UBE2V1 |
| 68 | GO_Biological_Process_2021 | negative regulation of cellular amide metaboli... | 8/86 | 3.142995e-04 | 1.217114e-02 | 0 | 0 | 5.134517 | 41.410720 | RBM4;PRNP;EIF4A3;DHX36;GZMB;YBX1;GAPDH;TOB1 |
| 69 | GO_Biological_Process_2021 | cellular response to zinc ion (GO:0071294) | 4/18 | 3.788618e-04 | 1.446170e-02 | 0 | 0 | 14.204496 | 111.907836 | MT2A;MT1F;MT1X;MT1E |
| 71 | GO_Biological_Process_2021 | regulation of RIG-I signaling pathway (GO:0039... | 4/19 | 4.723957e-04 | 1.739863e-02 | 0 | 0 | 13.256853 | 101.516916 | SEC14L1;BIRC2;OASL;BIRC3 |
| 73 | GO_Biological_Process_2021 | regulation of peptidyl-tyrosine phosphorylatio... | 8/92 | 4.972926e-04 | 1.795630e-02 | 0 | 0 | 4.766300 | 36.254063 | PRNP;PDCL3;TGFB1;SEMA4D;FYN;MIF;SAMSN1;CD44 |
| 74 | GO_Biological_Process_2021 | nucleic acid metabolic process (GO:0090304) | 7/71 | 5.215188e-04 | 1.857998e-02 | 0 | 0 | 5.465393 | 41.311624 | HNRNPL;PTBP1;DDX24;POLR2A;WTAP;KPNA2;HNRNPA0 |
| 76 | GO_Biological_Process_2021 | RNA splicing (GO:0008380) | 8/98 | 7.584158e-04 | 2.542252e-02 | 0 | 0 | 4.447179 | 31.949777 | IVNS1ABP;PPP2CA;PTBP1;SFPQ;RBM8A;SRSF2;BCAS2;S... |
| 78 | GO_Biological_Process_2021 | pyruvate metabolic process (GO:0006090) | 6/55 | 7.611532e-04 | 2.542252e-02 | 0 | 0 | 6.107768 | 43.857900 | LDHB;LDHA;PGK1;VDAC1;ALDOA;GAPDH |
| 79 | GO_Biological_Process_2021 | regulation of blood vessel endothelial cell mi... | 6/55 | 7.611532e-04 | 2.542252e-02 | 0 | 0 | 6.107768 | 43.857900 | MAP2K3;STAT5A;TGFB1;RGCC;CSNK2B;HIF1A |
itgae_ls[3].res2d.Term = itgae_ls[3].res2d.Term.str.split(" \(GO").str[0]
## dotplot
gseapy.dotplot(itgae_ls[3].res2d.iloc[[32,55,65,84],:],
size=50, top_term=10, title='Up',
cmap=make_colormap(['#f7f7f7', '#e5aca5']),
figsize=(3,5), vmin=1.3)
plt.savefig('figures/Nitgae_go_up.pdf')
plt.show()
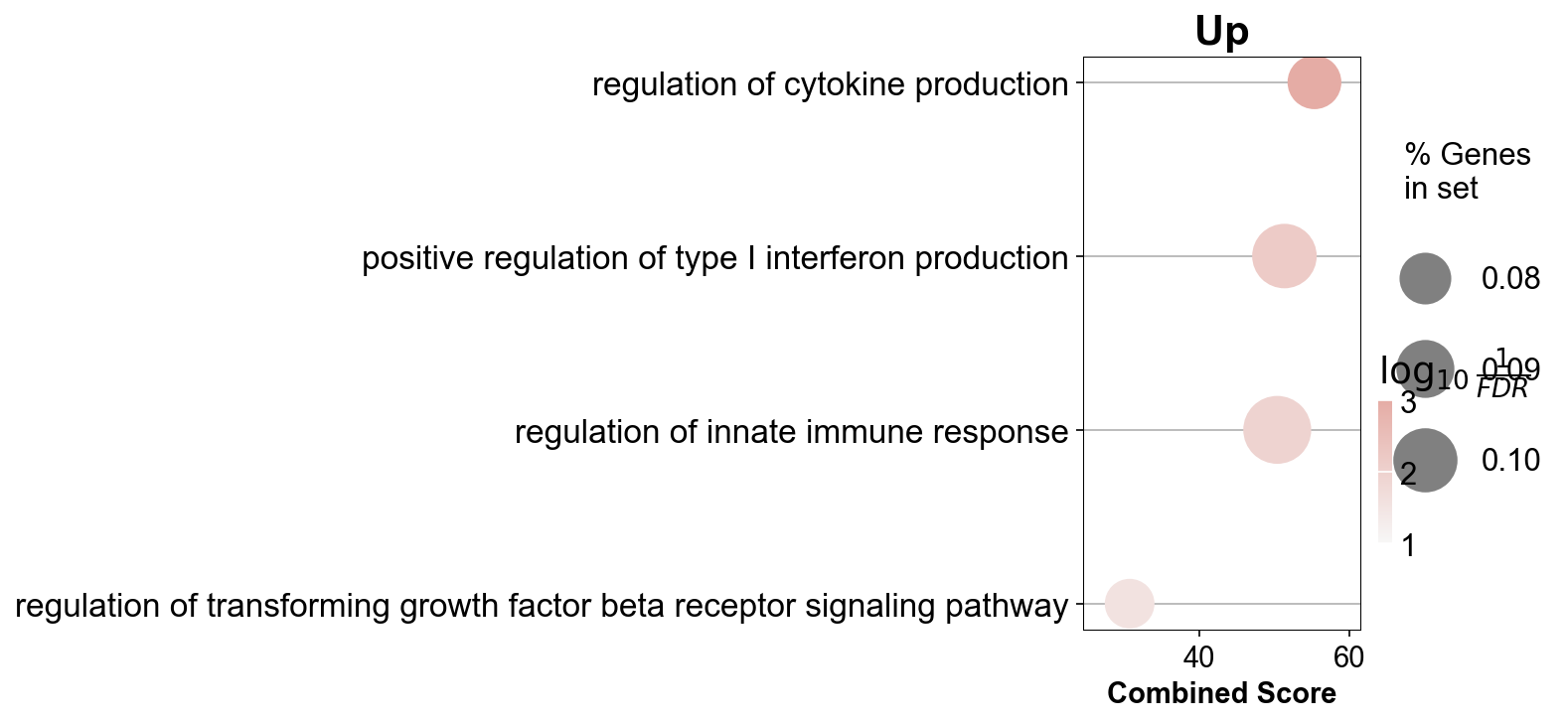
xbp_go_inter = xbp_go.append([gzmk_go,itgae_go])
xbp_go_inter = xbp_go_inter.drop_duplicates(['Term'], keep=False)
xbp_go_inter[0:50]
| Gene_set | Term | Overlap | P-value | Adjusted P-value | Old P-value | Old Adjusted P-value | Odds Ratio | Combined Score | Genes | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | GO_Biological_Process_2021 | protein transport (GO:0015031) | 45/369 | 3.924420e-10 | 3.580052e-07 | 0 | 0 | 3.178055 | 68.832322 | MACF1;COPB2;COPA;CLTC;USO1;MIA2;VPS26A;HSP90B1... |
| 4 | GO_Biological_Process_2021 | mitochondrial respiratory chain complex I asse... | 15/58 | 1.652156e-08 | 1.004786e-05 | 0 | 0 | 7.816083 | 140.053256 | NDUFB9;NDUFA8;NDUFB7;NDUFB10;NDUFA12;NDUFB5;ND... |
| 5 | GO_Biological_Process_2021 | NADH dehydrogenase complex assembly (GO:0010257) | 15/58 | 1.652156e-08 | 1.004786e-05 | 0 | 0 | 7.816083 | 140.053256 | NDUFB9;NDUFA8;NDUFB7;NDUFB10;NDUFA12;NDUFB5;ND... |
| 6 | GO_Biological_Process_2021 | cellular protein localization (GO:0034613) | 38/329 | 4.001543e-08 | 2.085947e-05 | 0 | 0 | 2.967994 | 50.556819 | CD63;COPB2;COPA;CLTC;USO1;VPS26A;MESD;GGA1;SNX... |
| 7 | GO_Biological_Process_2021 | mitochondrial ATP synthesis coupled electron t... | 16/71 | 4.625599e-08 | 2.109851e-05 | 0 | 0 | 6.521696 | 110.145419 | NDUFB9;NDUFA8;NDUFB7;NDUFB10;NDUFA12;NDUFB5;ND... |
| 8 | GO_Biological_Process_2021 | mitochondrial electron transport, NADH to ubiq... | 12/39 | 5.410310e-08 | 2.193580e-05 | 0 | 0 | 9.931644 | 166.179985 | NDUFB9;NDUFA8;NDUFB7;NDUFS8;NDUFB10;NDUFA12;ND... |
| 10 | GO_Biological_Process_2021 | aerobic electron transport chain (GO:0019646) | 15/70 | 2.463931e-07 | 8.173532e-05 | 0 | 0 | 6.106914 | 92.924868 | NDUFB9;NDUFA8;NDUFB7;NDUFB10;NDUFA12;NDUFB5;ND... |
| 12 | GO_Biological_Process_2021 | positive regulation of cellular component biog... | 17/92 | 3.815916e-07 | 1.071098e-04 | 0 | 0 | 5.082133 | 75.108416 | PDCD6IP;SMAD3;TGFB1;PIH1D1;VPS4B;PLEK;BRK1;PRK... |
| 14 | GO_Biological_Process_2021 | translational elongation (GO:0006414) | 18/104 | 4.843540e-07 | 1.178272e-04 | 0 | 0 | 4.695620 | 68.276427 | MRPS15;MRPS12;MRPS34;MRPS18B;MRPL28;MRPL36;EEF... |
| 15 | GO_Biological_Process_2021 | cellular protein modification process (GO:0006... | 78/1025 | 8.951947e-07 | 2.041603e-04 | 0 | 0 | 1.898475 | 26.438585 | ERO1A;GPAA1;PRKCSH;GMFG;PIK3CD;ILK;DCAF7;STK10... |
| 16 | GO_Biological_Process_2021 | retrograde transport, endosome to Golgi (GO:00... | 16/88 | 1.049169e-06 | 2.252010e-04 | 0 | 0 | 4.977412 | 68.526583 | TMED9;CLTC;VPS26A;SNX3;GGA1;LMAN1;SNX1;VPS51;T... |
| 17 | GO_Biological_Process_2021 | mitochondrial translational elongation (GO:007... | 16/89 | 1.228316e-06 | 2.359014e-04 | 0 | 0 | 4.908971 | 66.810438 | MRPS15;MRPS12;MRPS34;MRPS18B;MRPL28;MRPL36;MRP... |
| 18 | GO_Biological_Process_2021 | mitochondrial translational termination (GO:00... | 16/89 | 1.228316e-06 | 2.359014e-04 | 0 | 0 | 4.908971 | 66.810438 | MRPS15;MRPS12;MRPS34;MRPS18B;MRPL28;MRPL36;MRP... |
| 19 | GO_Biological_Process_2021 | mitochondrial respiratory chain complex assemb... | 16/90 | 1.434568e-06 | 2.617370e-04 | 0 | 0 | 4.842379 | 65.152503 | NDUFB9;COA3;NDUFA8;NDUFB7;NDUFB10;NDUFA12;NDUF... |
| 20 | GO_Biological_Process_2021 | protein-containing complex assembly (GO:0065003) | 30/267 | 1.892660e-06 | 3.288722e-04 | 0 | 0 | 2.857705 | 37.657481 | CDC123;STOML2;KMT2A;GPAA1;TUBGCP2;TRADD;USO1;W... |
| 21 | GO_Biological_Process_2021 | cytosolic transport (GO:0016482) | 18/116 | 2.515784e-06 | 4.150968e-04 | 0 | 0 | 4.118050 | 53.093714 | VPS13C;CLTC;VPS13A;VPS26A;SNF8;GGA1;SNX1;VPS51... |
| 22 | GO_Biological_Process_2021 | mitochondrial translation (GO:0032543) | 17/105 | 2.616395e-06 | 4.150968e-04 | 0 | 0 | 4.328409 | 55.636128 | MRPS15;MRPS12;MRPS34;MRPS18B;MRPL28;MRPL36;MTI... |
| 23 | GO_Biological_Process_2021 | regulation of autophagy (GO:0010506) | 27/231 | 2.896170e-06 | 4.403385e-04 | 0 | 0 | 2.982511 | 38.033336 | PLEKHF1;ROCK1;CISD2;VPS26A;MTDH;CAPNS1;RB1CC1;... |
| 25 | GO_Biological_Process_2021 | translational termination (GO:0006415) | 16/96 | 3.470882e-06 | 4.871250e-04 | 0 | 0 | 4.477791 | 56.290764 | MRPS15;MRPS12;MRPS34;MRPS18B;MRPL28;MRPL36;MRP... |
| 26 | GO_Biological_Process_2021 | peptidyl-serine phosphorylation (GO:0018105) | 21/156 | 3.987270e-06 | 5.388721e-04 | 0 | 0 | 3.493197 | 43.428833 | SRPK2;MAP4K1;MAP2K2;CAB39;ROCK1;PRKCB;PRKDC;PR... |
| 27 | GO_Biological_Process_2021 | intracellular protein transport (GO:0006886) | 34/336 | 4.275271e-06 | 5.571594e-04 | 0 | 0 | 2.545074 | 31.463888 | COPB2;COPA;CLTC;USO1;VPS26A;GGA1;LMAN1;SNX1;TP... |
| 30 | GO_Biological_Process_2021 | T cell activation (GO:0042110) | 15/92 | 9.200493e-06 | 1.082987e-03 | 0 | 0 | 4.357051 | 50.525472 | TNFSF14;PIK3CD;MSN;NLRC3;RASGRP1;DDOST;DPP4;ZA... |
| 32 | GO_Biological_Process_2021 | endocytic recycling (GO:0032456) | 11/52 | 1.127685e-05 | 1.246947e-03 | 0 | 0 | 5.983930 | 68.173466 | ARL4C;SNX3;EHD1;VPS51;SNX17;VPS35;VPS26A;SNF8;... |
| 33 | GO_Biological_Process_2021 | protein localization to membrane (GO:0072657) | 23/195 | 1.331277e-05 | 1.428774e-03 | 0 | 0 | 3.004126 | 33.726684 | PLEKHF1;ITGA4;ROCK1;CD81;TESC;ITGB2;ITGAL;TNF;... |
| 34 | GO_Biological_Process_2021 | homotypic cell-cell adhesion (GO:0034109) | 10/44 | 1.442079e-05 | 1.457168e-03 | 0 | 0 | 6.554671 | 73.063865 | PLEK;GNAS;ILK;FLNA;MYH9;TLN1;CLIC1;MYL12A;CD99... |
| 37 | GO_Biological_Process_2021 | platelet aggregation (GO:0070527) | 9/36 | 1.697354e-05 | 1.605428e-03 | 0 | 0 | 7.422688 | 81.529731 | PLEK;GNAS;ILK;FLNA;MYH9;TLN1;CLIC1;MYL12A;FERMT3 |
| 40 | GO_Biological_Process_2021 | membrane raft organization (GO:0031579) | 6/15 | 2.331139e-05 | 2.074713e-03 | 0 | 0 | 14.807588 | 157.946154 | ANXA2;NAXE;FLOT1;PPT1;FLOT2;S100A10 |
| 41 | GO_Biological_Process_2021 | post-Golgi vesicle-mediated transport (GO:0006... | 14/89 | 2.758841e-05 | 2.396907e-03 | 0 | 0 | 4.170567 | 43.783088 | VPS13C;CLTC;VPS13A;CLTA;SORL1;SCAMP3;BLOC1S4;R... |
| 42 | GO_Biological_Process_2021 | pinocytosis (GO:0006907) | 5/10 | 3.178643e-05 | 2.636106e-03 | 0 | 0 | 22.190255 | 229.812738 | PYCARD;MAPKAPK3;PPT1;DOCK2;SNX5 |
| 44 | GO_Biological_Process_2021 | positive regulation of cellular protein metabo... | 15/102 | 3.274641e-05 | 2.655370e-03 | 0 | 0 | 3.854217 | 39.801412 | CYFIP2;TGFB1;PRKDC;MSN;RPS27L;UPF3A;EEF2;CASP8... |
| 45 | GO_Biological_Process_2021 | positive regulation of transport (GO:0051050) | 14/91 | 3.563529e-05 | 2.826808e-03 | 0 | 0 | 4.061814 | 41.601806 | DYNC1H1;ANXA1;ANXA2;KMT2A;CHCHD10;CYBA;TNF;PYC... |
| 47 | GO_Biological_Process_2021 | peptidyl-serine modification (GO:0018209) | 20/169 | 4.555246e-05 | 3.442080e-03 | 0 | 0 | 3.008486 | 30.074773 | SRPK2;MAP4K1;PARP1;CAB39;ROCK1;PRKCB;PRKDC;PRK... |
| 48 | GO_Biological_Process_2021 | cellular protein catabolic process (GO:0044257) | 11/60 | 4.662983e-05 | 3.442080e-03 | 0 | 0 | 5.004864 | 49.914857 | CASP8;GZMA;CLTC;CAPN2;AP2S1;MDM2;CLTA;GZMB;CTS... |
| 49 | GO_Biological_Process_2021 | positive regulation of cellular process (GO:00... | 49/625 | 4.800483e-05 | 3.442080e-03 | 0 | 0 | 1.929870 | 19.191031 | CD81;TNF;CCAR1;MECP2;PYCARD;CAPNS1;RPS19;RPS6K... |
| 50 | GO_Biological_Process_2021 | membrane raft assembly (GO:0001765) | 4/6 | 4.905129e-05 | 3.442080e-03 | 0 | 0 | 44.336037 | 439.930715 | ANXA2;FLOT1;FLOT2;S100A10 |
| 51 | GO_Biological_Process_2021 | positive regulation of granulocyte differentia... | 4/6 | 4.905129e-05 | 3.442080e-03 | 0 | 0 | 44.336037 | 439.930715 | HAX1;TESC;HCLS1;EVI2B |
| 52 | GO_Biological_Process_2021 | endoplasmic reticulum to Golgi vesicle-mediate... | 21/185 | 5.511899e-05 | 3.794890e-03 | 0 | 0 | 2.871108 | 28.154131 | COPB2;DYNC1H1;COPA;TMED9;TRAPPC1;DCTN2;KDELR1;... |
| 53 | GO_Biological_Process_2021 | regulation of T cell apoptotic process (GO:007... | 5/11 | 5.619272e-05 | 3.797171e-03 | 0 | 0 | 18.490913 | 180.965448 | LGALS3;PRELID1;CCL5;PRKCQ;JAK3 |
| 55 | GO_Biological_Process_2021 | protein phosphorylation (GO:0006468) | 41/496 | 6.211135e-05 | 4.020727e-03 | 0 | 0 | 2.037618 | 19.737554 | ROCK1;CAB39;PRKDC;GMFG;PIK3CD;ILK;STK10;RPS6KA... |
| 56 | GO_Biological_Process_2021 | regulation of cell adhesion (GO:0030155) | 17/133 | 6.326954e-05 | 4.020727e-03 | 0 | 0 | 3.278793 | 31.699721 | RIPOR2;PPP1R12A;ROCK1;TESC;PDE3B;ADAM10;PRKCA;... |
| 58 | GO_Biological_Process_2021 | regulation of spindle assembly (GO:0090169) | 7/25 | 6.797320e-05 | 4.188690e-03 | 0 | 0 | 8.643734 | 82.948702 | SENP6;DYNC1H1;PDCD6IP;VPS4B;TPR;CHMP2A;CHMP4B |
| 59 | GO_Biological_Process_2021 | phosphorylation (GO:0016310) | 35/400 | 6.887405e-05 | 4.188690e-03 | 0 | 0 | 2.163066 | 20.729165 | ROCK1;PRKDC;GMFG;PIK3CD;ILK;STK10;RPS6KA5;GRK2... |
| 60 | GO_Biological_Process_2021 | proteolysis involved in cellular protein catab... | 12/74 | 7.521338e-05 | 4.435290e-03 | 0 | 0 | 4.317148 | 40.992101 | DDB1;CASP8;PDCD6IP;VPS4B;GZMA;CAPN2;MDM2;CHMP4... |
| 62 | GO_Biological_Process_2021 | leukocyte tethering or rolling (GO:0050901) | 6/18 | 7.730898e-05 | 4.477785e-03 | 0 | 0 | 11.103949 | 105.128862 | SPN;SELPLG;ITGA4;ROCK1;ITGB7;TNF |
| 63 | GO_Biological_Process_2021 | IRE1-mediated unfolded protein response (GO:00... | 10/53 | 7.960779e-05 | 4.538888e-03 | 0 | 0 | 5.180321 | 48.893932 | DNAJC3;ERN1;MYDGF;ACADVL;XBP1;SSR1;TPP1;TLN1;P... |
| 67 | GO_Biological_Process_2021 | cytoplasmic translational initiation (GO:0002183) | 6/19 | 1.088592e-04 | 5.679776e-03 | 0 | 0 | 10.249263 | 93.529190 | MCTS1;EIF3M;EIF2S2;EIF3A;EIF4B;EIF4G1 |
| 68 | GO_Biological_Process_2021 | leukocyte adhesion to vascular endothelial cel... | 6/19 | 1.088592e-04 | 5.679776e-03 | 0 | 0 | 10.249263 | 93.529190 | SPN;SELPLG;ITGA4;ROCK1;ITGB7;TNF |
| 69 | GO_Biological_Process_2021 | death-inducing signaling complex assembly (GO:... | 4/7 | 1.105136e-04 | 5.679776e-03 | 0 | 0 | 29.555813 | 269.264455 | CASP8;TRADD;TNF;TNFRSF1A |
| 70 | GO_Biological_Process_2021 | regulation of myosin-light-chain-phosphatase a... | 4/7 | 1.105136e-04 | 5.679776e-03 | 0 | 0 | 29.555813 | 269.264455 | PPP1R12A;ROCK1;NCKAP1L;TNF |
| 72 | GO_Biological_Process_2021 | vascular endothelial growth factor receptor si... | 11/67 | 1.326708e-04 | 6.631723e-03 | 0 | 0 | 4.377649 | 39.082076 | CYFIP2;MAPKAPK3;ROCK1;NCF4;PXN;BRK1;CYBA;NCKAP... |
xbp_ls[3].res2d.Term = xbp_ls[3].res2d.Term.str.split(" \(GO").str[0]
## dotplot
gseapy.dotplot(xbp_ls[3].res2d.iloc[[23,56,68,86],:],
size=20, top_term=20, title='Up', vmin=1.3,
cmap=make_colormap(['#dee0c1','#949b0d']),
figsize=(3,5))
plt.savefig('figures/XBP1_go_up.pdf')
plt.show()
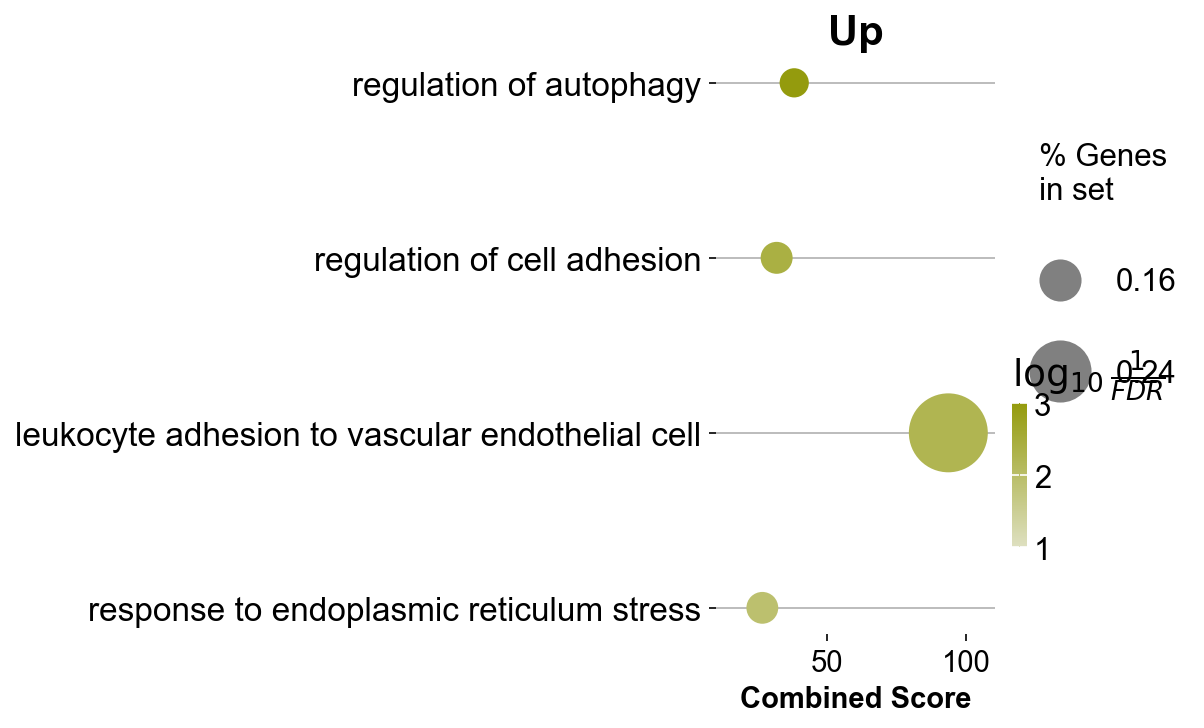
gzmk_final = gzmk_ls[3].res2d.iloc[[0,18,21,52],:]
itgae_final = itgae_ls[3].res2d.iloc[[32,55,65,84],:]
xbp_final = xbp_ls[3].res2d.iloc[[23,56,68,86],:]
fig2_goterm = gzmk_final.append([itgae_final,xbp_final])
fig2_goterm.to_csv('tables/fig2_goterm.csv', index=0)
Clonotype Sharing Analysis¶
clone_stumor = clone_subtype[(clone_subtype['disease_type']=='Solid tumor') &
(clone_subtype['meta_cell_subset']=='normal_CD8')]
cell_subtypes_mod = [
'GZMK+ Tex',
'ITGAE+ Tex',
'XBP1+ Tex',
'Tn',
'Tcm',
'Early Tcm/Tem',
'GZMK+ Tem',
'GNLY+ Temra',
'CMC1+ Temra',
'ZNF683+ Teff',
'MAIT',
'ILTCK',
'ITGAE+ Trm',
'CREM+ Trm',
'ITGB2+ Trm',
'Tpex',
'MACF1+ T',
'Cycling T'
]
stumor_circos = circos_edges(clone_stumor,
subtypes=cell_subtypes_mod,
file_name='figures/stumor_clonotype.csv')
Venn3¶
stumor_sanke, stumor_sanke_venn = sanke_plot3(
stumor_circos,
column1='GZMK+ Tex',
column2='ITGAE+ Tex',
column3='XBP1+ Tex',
file_name='figures/stumor_sanke3.csv')
stumor_sanke
| source | target | value | sg | tg | value_raw | |
|---|---|---|---|---|---|---|
| 3 | GZMK+ Tex | Tcm | 0.161551 | 0 | 1 | 1 |
| 5 | GZMK+ Tex | GZMK+ Tem | 14.539580 | 0 | 1 | 90 |
| 6 | GZMK+ Tex | GNLY+ Temra | 0.807754 | 0 | 1 | 5 |
| 7 | GZMK+ Tex | CMC1+ Temra | 7.592892 | 0 | 1 | 47 |
| 8 | GZMK+ Tex | ZNF683+ Teff | 0.484653 | 0 | 1 | 3 |
| 9 | GZMK+ Tex | MAIT | 0.323102 | 0 | 1 | 2 |
| 10 | GZMK+ Tex | ILTCK | 0.323102 | 0 | 1 | 2 |
| 11 | GZMK+ Tex | ITGAE+ Trm | 1.938611 | 0 | 1 | 12 |
| 12 | GZMK+ Tex | CREM+ Trm | 26.978998 | 0 | 1 | 167 |
| 13 | GZMK+ Tex | ITGB2+ Trm | 44.749596 | 0 | 1 | 277 |
| 14 | GZMK+ Tex | Tpex | 0.969305 | 0 | 1 | 6 |
| 15 | GZMK+ Tex | MACF1+ T | 1.130856 | 0 | 1 | 7 |
| 18 | ITGAE+ Tex | Tn | 0.323102 | 0 | 1 | 2 |
| 19 | ITGAE+ Tex | Tcm | 1.292407 | 0 | 1 | 8 |
| 20 | ITGAE+ Tex | Early Tcm/Tem | 1.615509 | 0 | 1 | 10 |
| 21 | ITGAE+ Tex | GZMK+ Tem | 1.130856 | 0 | 1 | 7 |
| 22 | ITGAE+ Tex | GNLY+ Temra | 4.361874 | 0 | 1 | 27 |
| 23 | ITGAE+ Tex | CMC1+ Temra | 2.907916 | 0 | 1 | 18 |
| 24 | ITGAE+ Tex | ZNF683+ Teff | 24.232633 | 0 | 1 | 150 |
| 25 | ITGAE+ Tex | MAIT | 0.969305 | 0 | 1 | 6 |
| 26 | ITGAE+ Tex | ILTCK | 0.161551 | 0 | 1 | 1 |
| 27 | ITGAE+ Tex | ITGAE+ Trm | 82.875606 | 0 | 1 | 513 |
| 28 | ITGAE+ Tex | CREM+ Trm | 25.040388 | 0 | 1 | 155 |
| 29 | ITGAE+ Tex | ITGB2+ Trm | 17.124394 | 0 | 1 | 106 |
| 30 | ITGAE+ Tex | Tpex | 0.161551 | 0 | 1 | 1 |
| 31 | ITGAE+ Tex | MACF1+ T | 8.885299 | 0 | 1 | 55 |
| 34 | XBP1+ Tex | Tcm | 0.646204 | 0 | 1 | 4 |
| 35 | XBP1+ Tex | Early Tcm/Tem | 1.453958 | 0 | 1 | 9 |
| 36 | XBP1+ Tex | GZMK+ Tem | 7.108239 | 0 | 1 | 44 |
| 37 | XBP1+ Tex | GNLY+ Temra | 1.130856 | 0 | 1 | 7 |
| 38 | XBP1+ Tex | CMC1+ Temra | 2.423263 | 0 | 1 | 15 |
| 39 | XBP1+ Tex | ZNF683+ Teff | 53.311793 | 0 | 1 | 330 |
| 40 | XBP1+ Tex | MAIT | 1.130856 | 0 | 1 | 7 |
| 42 | XBP1+ Tex | ITGAE+ Trm | 3.231018 | 0 | 1 | 20 |
| 43 | XBP1+ Tex | CREM+ Trm | 0.484653 | 0 | 1 | 3 |
| 44 | XBP1+ Tex | ITGB2+ Trm | 1.615509 | 0 | 1 | 10 |
stumor_sanke_venn
[619, 1059, 478, 449, 86, 82, 0]
venn3(subsets=stumor_sanke_venn,
set_labels=['GZMK+ Tex', 'ITGAE+ Tex', 'XBP1+ Tex'],
set_colors=['#082799', '#d67e09', '#6b7cad'])
plt.savefig('figures/stumor_venn3.pdf')
plt.show()
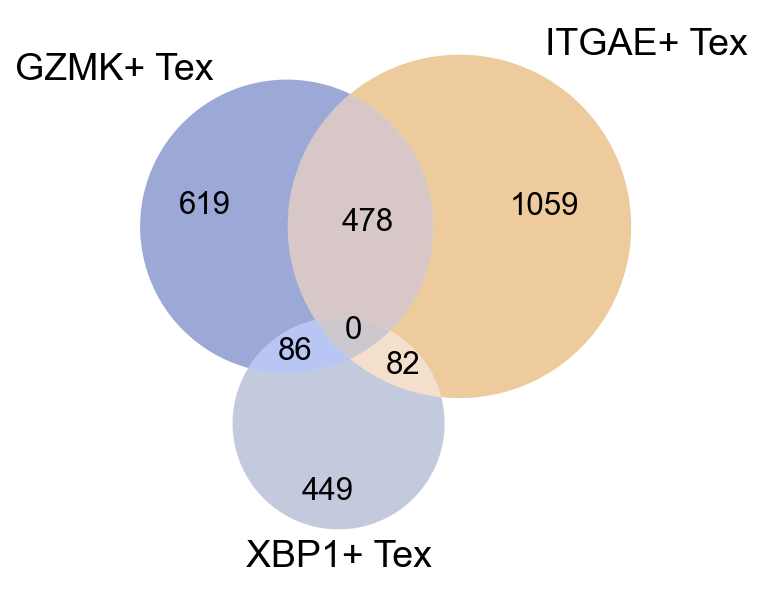
## Figure2E Stats and Plots
clone_id_name = {
'GZMK1_ccRCC':90249,
'GZMK2_NPC':34940,
'ITGAE1_NPC':103807,
'ITGAE2_NSCLC':425078,
'XBP1_PCC':251863,
'XBP2_PCC':251324,
}
for file_name, clone_id in zip(clone_id_name.keys(),clone_id_name.values()):
adata_nogex.obs['clone_umap'] = None
adata_nogex.obs.loc[
(
adata_nogex.obs['cc_aa_identity']==clone_id
), 'clone_umap'
] = adata_nogex.obs.cell_subtype_3
## Figure2E
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='clone_umap', palette=subtype_color,
size=100, na_color='#f3f3f3', ax=ax)
fig.savefig('figures/fig3_updated/single_clone_'+file_name+'.png', dpi=600)
subtype_used = np.unique(adata_nogex.obs[~adata_nogex.obs['clone_umap'].isna()]['clone_umap'])
subtype_used = np.setdiff1d(subtype_used, 'Cycling T')
clone_pie = tissue_pie_data(adata_use=adata_nogex,
groupby='atlas_name',
groupby_type='huARdbv2',
ratioby='clone_umap',
ratioby_type=subtype_used,
expanded=False)
clone_pie_dict = dict(zip(subtype_used, clone_pie[0][:-1]))
clone_pie_tuple = sorted(clone_pie_dict.items(), key=lambda item: item[1], reverse=True)
clone_pie_keys = [x[0] for x in clone_pie_tuple]
clone_pie_values = [x[1] for x in clone_pie_tuple]
fig,ax = plt.subplots(figsize=(5,3))
ax.pie(clone_pie_values, radius=1,
colors=list(map(subtype_color.get, clone_pie_keys)),
autopct='%1.0f%%', pctdistance=1.2)
fig.savefig('figures/fig3_updated/single_clone_'+file_name+'.pie.pdf')
plt.show()
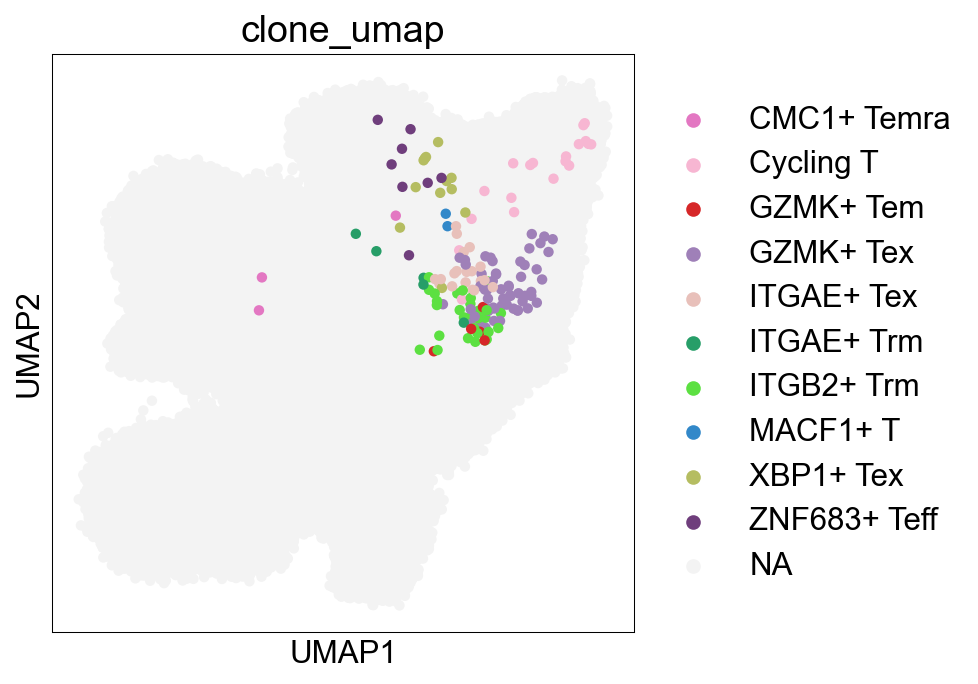
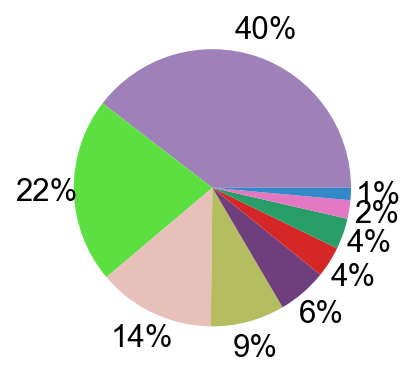
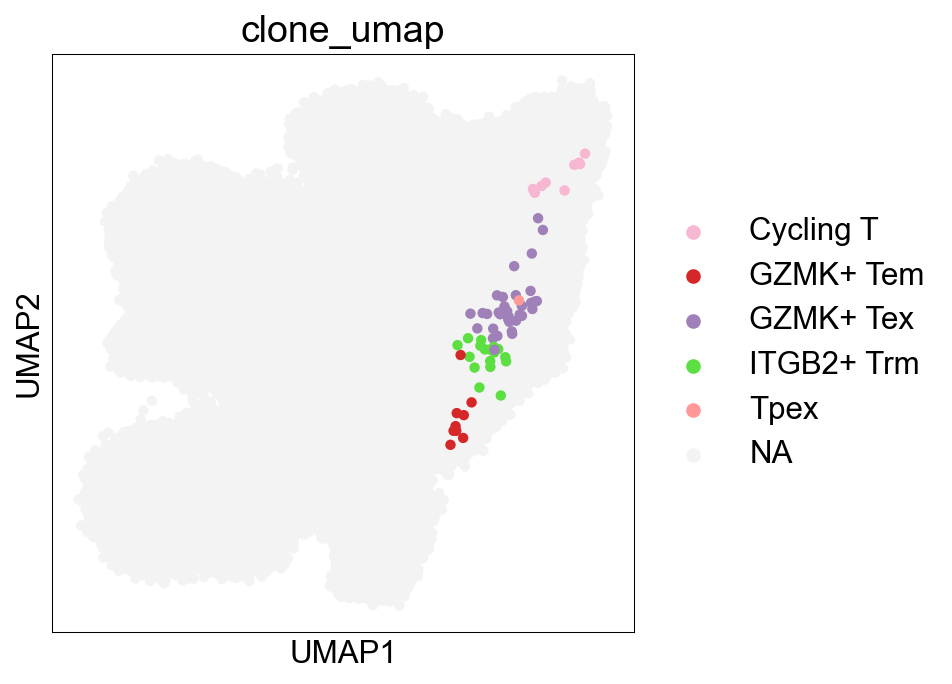
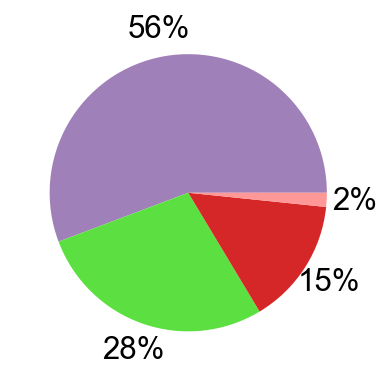
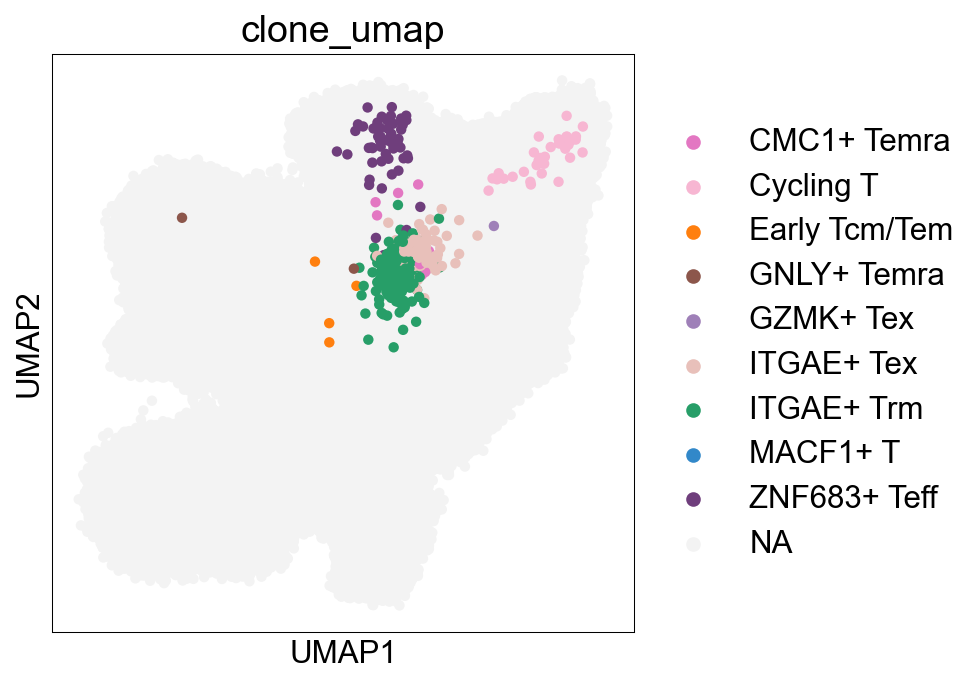
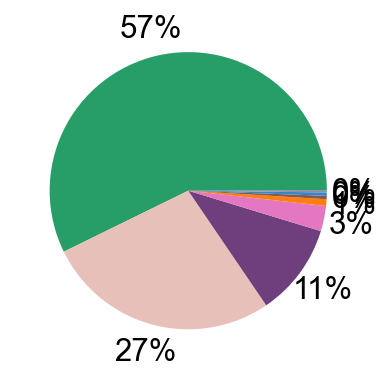
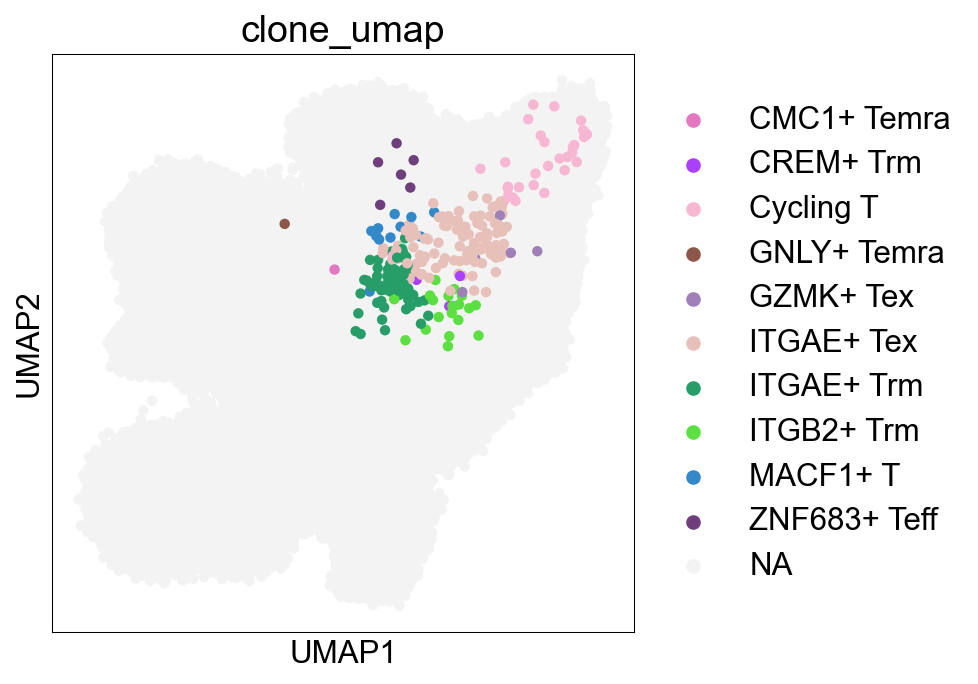
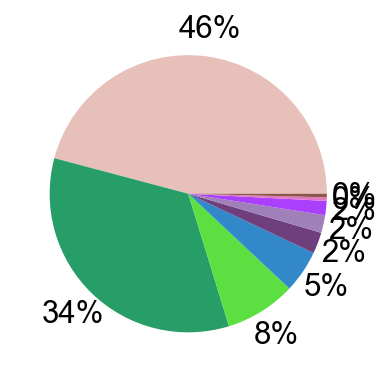
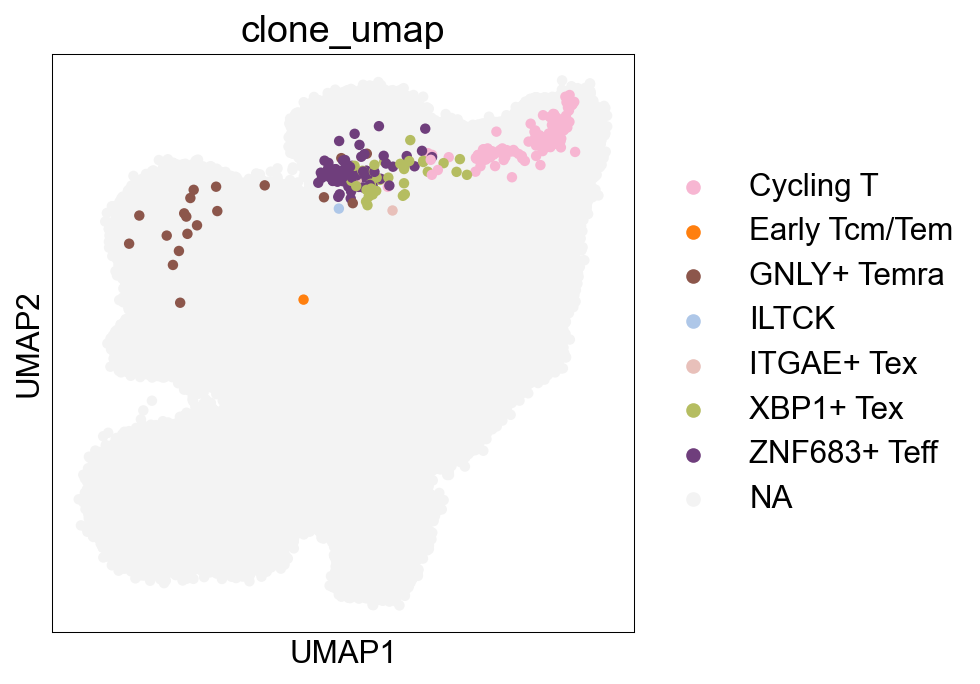
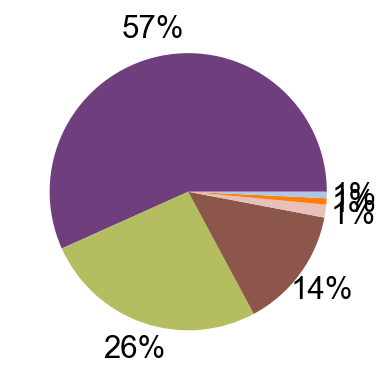
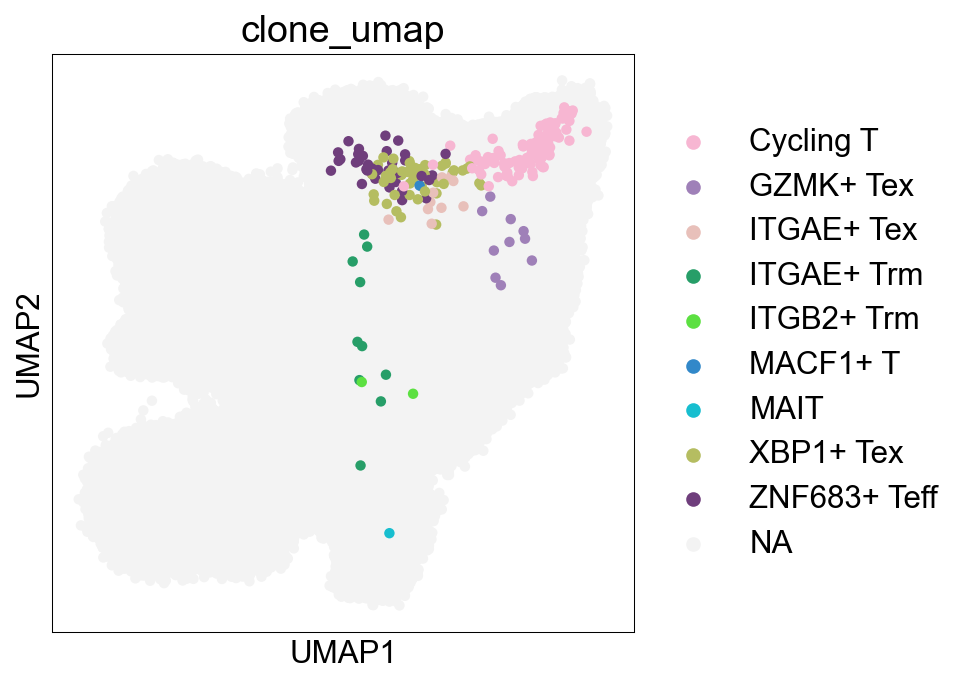
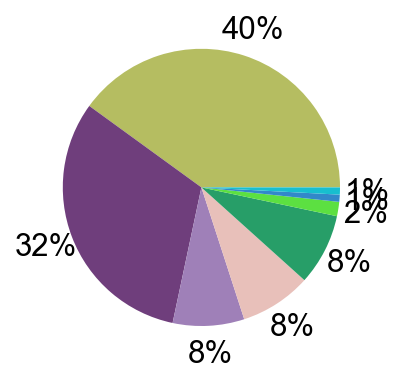
SupFigure9¶
Extended Data Figure 9A¶
sc.pl.umap(adata_nogex, color='CXCL13', cmap='Reds', size=5)
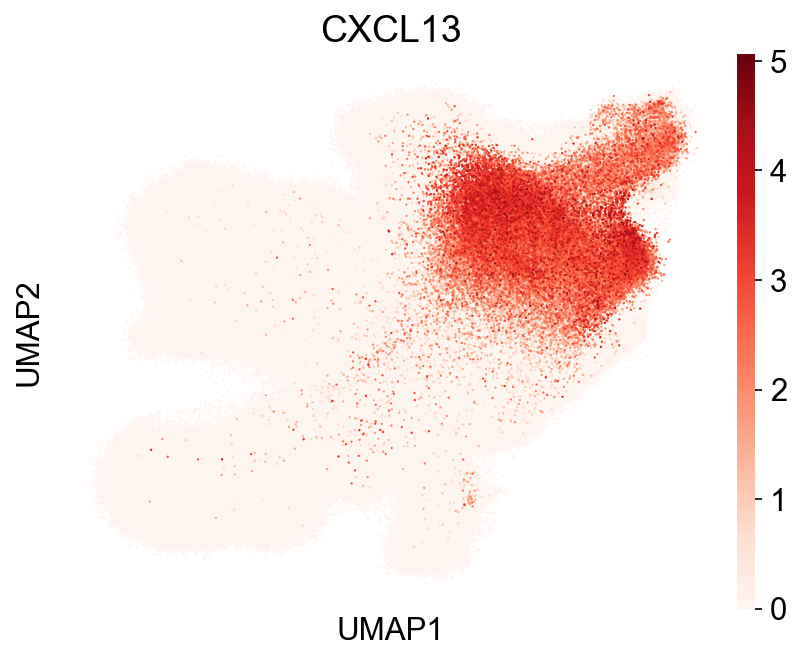
adata_ex = adata_ex[adata_ex.obs['disease']!='Organoid']
adata_ex.obs['disease_type'] = adata_ex.obs['disease_type'].cat.reorder_categories(disease_types)
adata_ex_nmal = adata_ex[adata_ex.obs['meta_cell_subset']=='normal_CD8']
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.violin(adata_ex_nmal, keys='CXCL13', stripplot=False,
groupby='disease_type', rotation=45, ax=ax)
fig.savefig('figures/CXCL13_disease.pdf')
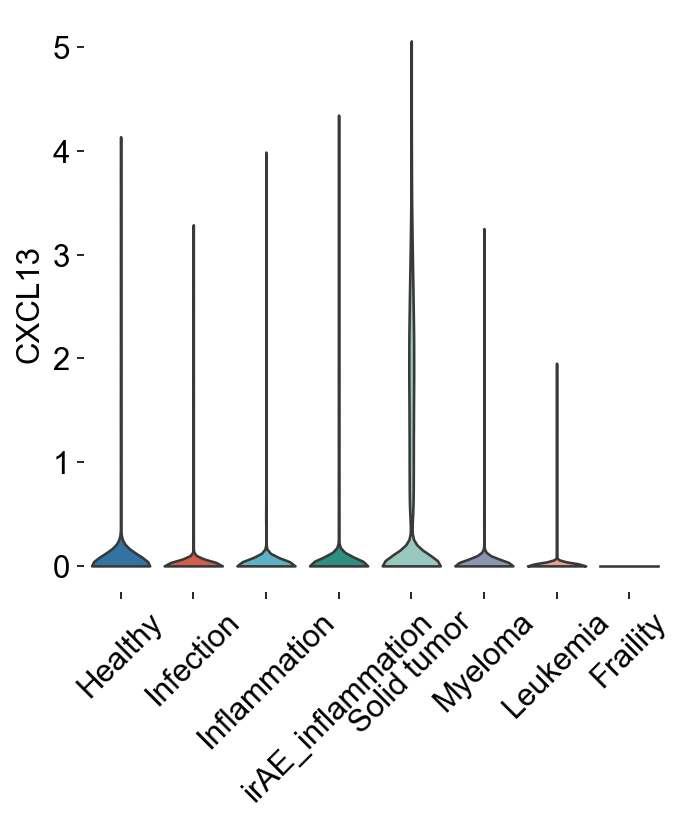
Extended Data Figure 9B-C¶
adata_nmal = adata_nogex[adata_nogex.obs['meta_cell_subset']=='normal_CD8']
adata_nmal_stumor = adata_nmal[adata_nmal.obs['disease_type']=='Solid tumor']
# adata_nmal_stumor_TIL = adata_nmal_stumor[adata_nmal_stumor.obs['meta_tissue_type_aggr']=='TIL']
obs = adata_nmal_stumor.obs
obs = obs[list(map(lambda x: 'Tex' in x, obs['cell_subtype_3']))]
# obs = obs[obs['study_name'].isin(list(map(lambda z: z[0], filter(lambda x: x[1] > 10, Counter(obs['study_name']).items()))))]
# obs = obs[obs['sample_name'].isin(list(map(lambda z: z[0], filter(lambda x: x[1] > 10, Counter(obs['sample_name']).items()))))]
sample_information = pd.read_csv("/Users/snow/Desktop/sample_information.csv", index_col=0)
obs['disease_type'] = list(sample_information.loc[obs['sample_name'],'Disease'])
obs['disease_type'] = obs['disease_type'].replace("Melanoma",'Skin cancer').replace("Basal cell carinoma tumor","Skin cancer").replace("Squamous cell carcinoma tumor","Skin cancer")
obs = obs[~(np.array(obs['individual_id'] == 'su010') & np.array(obs['disease_type'] == 'Basal cell carinoma tumor'))]
obs['disease_meta_type'] = list(sample_information.loc[obs['sample_name'],'Disease Metatype'])
# the order of disease
factors = {
'Breast cancer': 6.0,
'Cholangiocarcinoma': 7.0,
'Clear cell renal cell carcinoma': 10.0,
'Esophagus squamous cell carcinoma': 2.0,
'Gastric Cancer': 5.0,
'NSCLC': 4.0,
'Nasopharyngeal carcinoma': 9.0,
'Pancreatic Cancer': 1.0,
'Prostate Cancer': 8.0,
'Skin cancer': 3.0
}
obs = obs[obs['disease_meta_type'] == 'Solid tumor']
obs = obs[obs['disease_type'] != 'BEAM-T']
B = pd.DataFrame(
list(obs[list(map(lambda x: type(x) == str, obs['cell_subtype_3']))].groupby('disease_type').agg(
{'cell_subtype_3':lambda x: dict(Counter(x))
}).iloc[:,0])).fillna(0)
B.insert(0,'disease_type',pd.Categorical(obs['disease_type']).categories)
B['factors'] = list(map(factors.get, B['disease_type']))
B = B.sort_values("factors")
B = B.iloc[:,:-1]
B
B.plot(x='disease_type', kind='bar', stacked=True, color=subtype_color)
<Axes: xlabel='disease_type'>
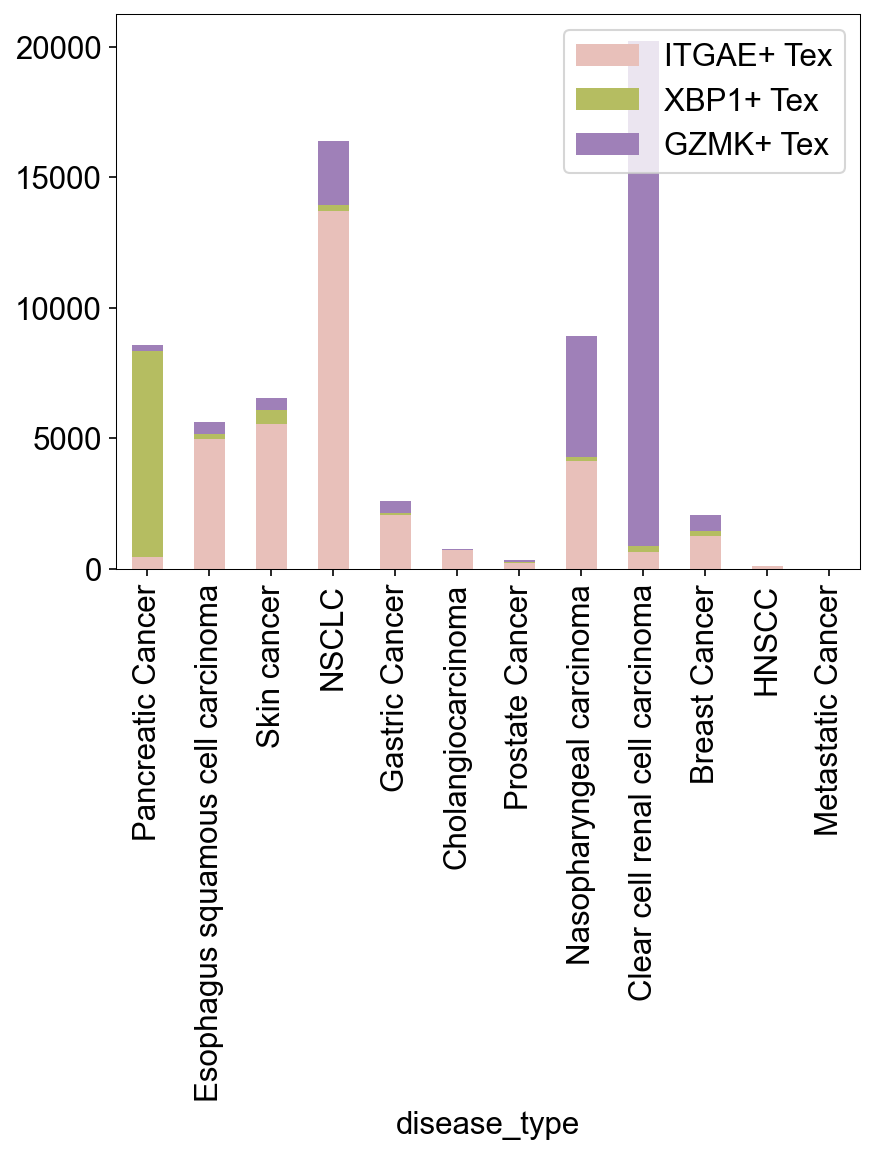
from matplotlib.patches import PathPatch
sample_information = pd.read_csv("/Users/snow/Desktop/sample_information.csv", index_col=0)
adata_nmal_stumor = sc.read_h5ad("/Volumes/rsch/GEXnotebook/source/adata_nmal_stumor_TIL.h5ad")
obs = adata_nmal_stumor.obs
obs['individual'] = list(sample_information.loc[obs['sample_name'], 'Individual ID'])
obs['disease_type'] = obs['disease']
obs['disease_type'] = obs['disease_type'].replace("Melanoma",'Skin cancer').replace("Basal cell carinoma tumor","Skin cancer").replace("Squamous cell carcinoma tumor","Skin cancer")
obs = obs[~(np.array(obs['individual'] == 'su010') & np.array(obs['disease_type'] == 'Basal cell carinoma tumor'))]
obs['individual'] = list(sample_information.loc[obs['sample_name'],'Individual ID'])
# obs = obs[obs['study_name'].isin(list(map(lambda z: z[0], filter(lambda x: x[1] > 10, Counter(obs['study_name']).items()))))]
obs = obs[obs['individual'].isin(list(map(lambda z: z[0], filter(lambda x: x[1] > 100, Counter(obs['individual']).items()))))]
factors2 = {
'ITGAE+ Tex': 1,
'GZMK+ Tex': 0,
'XBP1+ Tex': 2
}
obs = obs[obs['cell_subtype_3'].isin(factors2.keys())]
sample_information = pd.read_csv("/Users/snow/Desktop/sample_information.csv", index_col=0)
obs['disease_meta_type'] = list(sample_information.loc[obs['sample_name'],'Disease Metatype'])
sample_information = pd.read_csv("/Users/snow/Desktop/sample_information.csv", index_col=0)
obs = obs[obs['disease_meta_type'] == 'Solid tumor']
c = Counter(obs['individual'])
agg = obs.groupby(['disease_type','individual',"cell_subtype_3"]).agg({'individual': lambda x: len(x) / c[list(x)[0]]})
agg.columns=['count']
agg = pandas_aggregation_to_wide(agg[~agg.iloc[:,0].isna()])
agg = agg[agg['cell_subtype_3'].isin(factors2.keys())]
#s = set(list(map(lambda x: x[0], list(filter(lambda x: x[1] >= 3*3, Counter(agg['disease_type']).items())))))
#agg = agg[list(map(lambda x: x in s, agg['disease_type']))]
_d = agg.groupby(["individual",'disease_type']).agg({'cell_subtype_3':lambda x: len(np.unique(x)) == 3})
_d = set(list(map(lambda x: x[0], _d[_d.iloc[:,0]].index)))
agg = agg[list(map(lambda x: x in _d, agg['individual']))]
p = agg.pivot_table(values='count',index='disease_type',columns='cell_subtype_3').fillna(0)
factors = {v:k for k,v in enumerate(np.array(p.index)[sns.clustermap(
p
).dendrogram_row.reordered_ind])}
plt.close()
factors = dict(zip(p.index,(p['GZMK+ Tex'].rank())))
agg['disease_type_key'] = list(map(factors.get, agg['disease_type']))
agg['cell_subtype_3_prediction_key'] = list(map(lambda x: factors2.get(x), agg['cell_subtype_3']))
fig,ax=createFig(figsize=(14,4))
print(Counter(agg['disease_type']))
palette={
'GZMK+ Tex':'#C5B0D5',
'GZMK+ Tex DUSP1+': '#2CD500',
'GZMK+ Tex IL7R+': '#00B4DA',
'GZMK+ Tex ISG+': '#D5006F',
'GZMK+ Tex TNFRSF9+': '#9500A3',
'ITGAE+ Tex':'#E8C0BA',
'ITGAE+ Tex DUSP1+': '#1A7C00',
'ITGAE+ Tex IL7R+': '#0065DA',
'ITGAE+ Tex ISG+': '#D51A00',
'XBP1+ Tex':'#b5bd61',
}
sns.boxplot(data=agg.sort_values("cell_subtype_3").sort_values("disease_type_key"), hue='cell_subtype_3', x='disease_type', y='count', showfliers=False, ax=ax, palette=palette)
s1=set(list(map(str,ax.get_children())))
stripplot = sns.stripplot(data=agg.sort_values("cell_subtype_3").sort_values("disease_type_key"), hue='cell_subtype_3', x='disease_type', y='count', dodge=True, palette=palette)
s2=set(list(map(str,ax.get_children())))
s3 = s2.difference(s1)
artists = list(filter(lambda artist:isinstance(artist, matplotlib.collections.PathCollection) and str(artist) in s3, stripplot.get_children()))
for i in range(0,len(artists), 3):
x1 = artists[i].get_offsets()[:, 0]
y1 = artists[i].get_offsets()[:, 1]
x2 = artists[i+1].get_offsets()[:, 0]
y2 = artists[i+1].get_offsets()[:, 1]
x3 = artists[i+2].get_offsets()[:, 0]
y3 = artists[i+2].get_offsets()[:, 1]
ax.plot([x1,x2],[y1,y2],linestyle='-', marker='', color='#D4D4D4')
ax.plot([x2,x3],[y2,y3],linestyle='-', marker='', color='#D4D4D4')
ax.spines['right'].set_visible(False)
ax.spines['top'].set_visible(False)
adjust_box_widths(fig, 0.6)
plt.xticks(rotation=90)
# plt.savefig("./figures/20240123_tumor_tex_cell_type_composition.pdf")
Counter({'Skin cancer': 39, 'Clear cell renal cell carcinoma': 30, 'Nasopharyngeal carcinoma': 30, 'Breast cancer': 27, 'Gastric Cancer': 24, 'Esophagus squamous cell carcinoma': 21, 'Pancreatic Cancer': 18, 'NSCLC': 15, 'Prostate Cancer': 15, 'Cholangiocarcinoma': 6})
([0, 1, 2, 3, 4, 5, 6, 7, 8, 9],
[Text(0, 0, 'Pancreatic Cancer'),
Text(1, 0, 'Esophagus squamous cell carcinoma'),
Text(2, 0, 'Skin cancer'),
Text(3, 0, 'NSCLC'),
Text(4, 0, 'Gastric Cancer'),
Text(5, 0, 'Breast cancer'),
Text(6, 0, 'Cholangiocarcinoma'),
Text(7, 0, 'Prostate Cancer'),
Text(8, 0, 'Nasopharyngeal carcinoma'),
Text(9, 0, 'Clear cell renal cell carcinoma')])
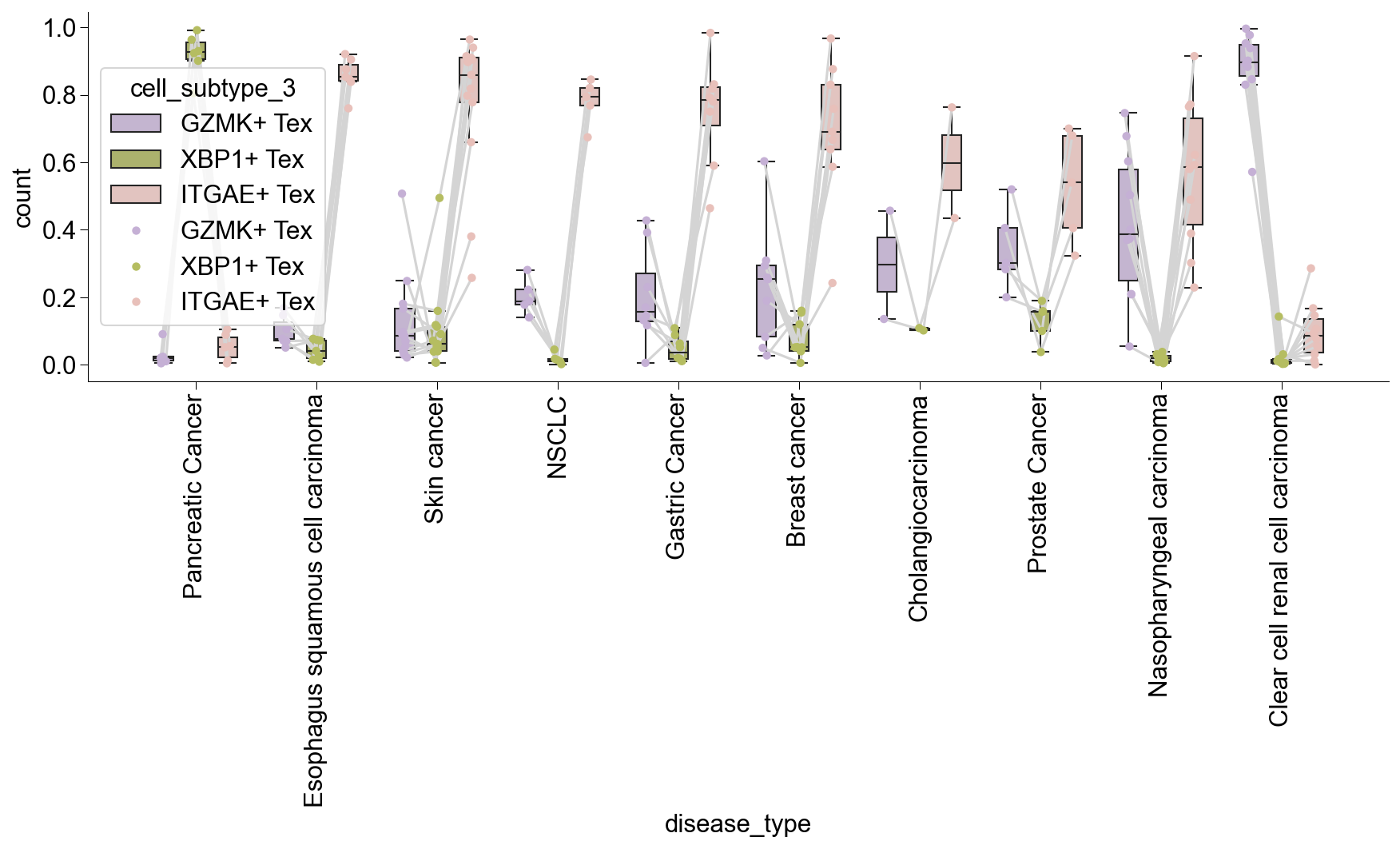
Part3ExpansionInInflam&irAE¶
SupColitisVsArthritis¶
adata_irae_arth = adata_nogex[adata_nogex.obs['study_name'].str.contains('Kim_2022')]
adata_irae_arth_circ = adata_irae_arth[adata_irae_arth.obs['meta_tissue_type_aggr'] == 'Circulating']
adata_irae_arth_tissue = adata_irae_arth[adata_irae_arth.obs['meta_tissue_type'].str.contains('Tissue')]
adata_irae_colitis = adata_nogex[adata_nogex.obs['disease'].str.contains("CPI-colitis")]
target_cells = 8486
cluster_key = 'disease'
adata = sc.concat([adata_irae_colitis, adata_irae_arth_tissue]) ## minimum #cell=9400
adatas = [adata[adata.obs[cluster_key]==clust] for clust in np.unique(adata.obs[cluster_key])]
for dat in adatas:
if dat.n_obs > target_cells:
sc.pp.subsample(dat, n_obs=target_cells)
adata_downsampled = adatas[0].concatenate(*adatas[1:])
adata_downsampled.obs.index = [ '-'.join(i.split('-')[0:3]) for i in adata_downsampled.obs.index ]
adata_nogex.obs['expanded_distribution'] = None
adata_nogex.obs.loc[
adata_downsampled.obs[
adata_downsampled.obs['cc_aa_identity']>=3
].index, 'expanded_distribution'
] = adata_downsampled.obs['disease']
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_nogex, color='expanded_distribution', size=10, na_color='#f7f7f7',
ax=ax, palette=['#ff707e','#3f5070'])
fig.savefig('figures/B_arth_colitis.png', dpi=600)
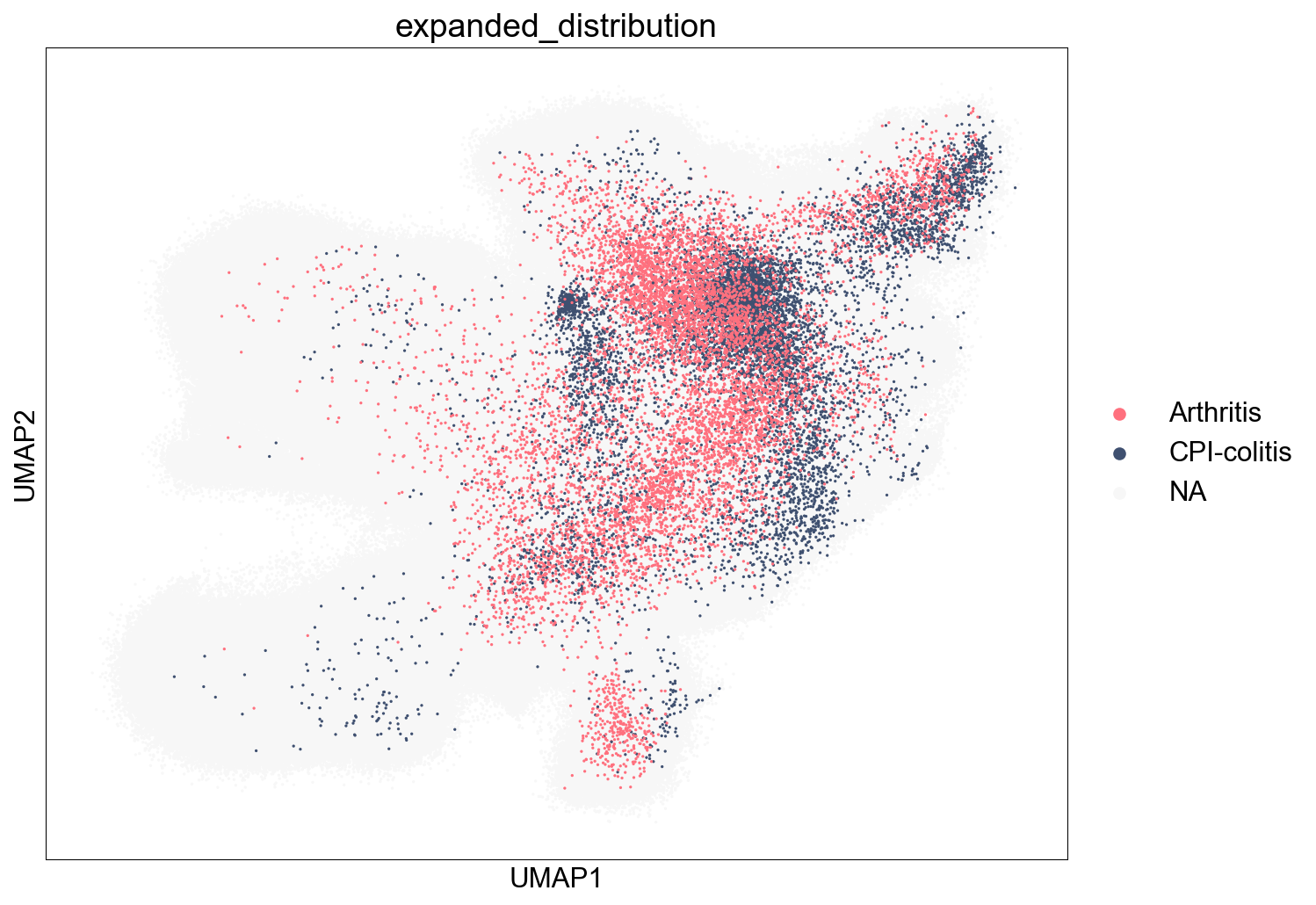
arth_tissue_expansion_pie = tissue_pie_data(adata_use=adata_irae_arth_tissue,
groupby='disease_type', groupby_type='irAE_inflammation',
ratioby='expansion_size', ratioby_type=['1', '2'],
expanded=False)
arth_tissue_subtypes = cell_subtypes[1:]
arth_ratio = tissue_pie_data(adata_use=adata_hiit_tissue_arth,
groupby='disease', groupby_type='Arthritis',
ratioby='cell_subtype_3', ratioby_type=arth_tissue_subtypes,
expanded=True)
fig,ax = plt.subplots(1,2)
ax[0].pie(arth_tissue_expansion_pie[0], radius=1, colors=expansion_size_color,
autopct='%1.0f%%', pctdistance=1.2, explode=(0,0,0.1), shadow=True,startangle=90)
ax[1].pie(arth_ratio[0], radius=1, colors=list(subtype_color.values())[1:],
autopct='%1.0f%%', pctdistance=1.2)
fig.savefig('figures/C_rth_pie.pdf')
plt.show()
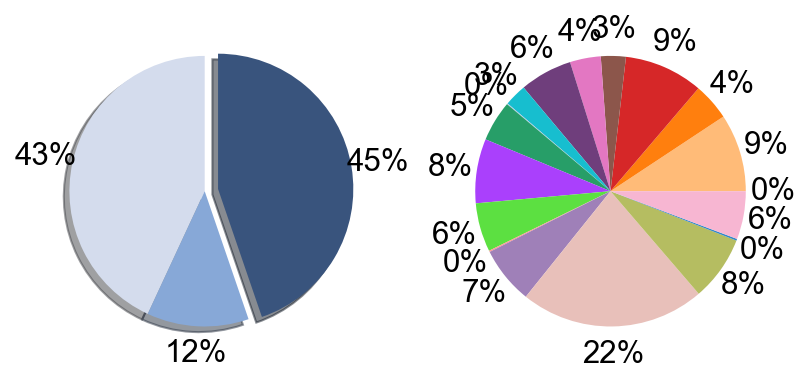
Part4SharingInInflam&irAE¶
DataProcessing¶
clone_circ = clone_df_generate(adata_hiit_circ)
clone_tissue = clone_df_generate(adata_hiit_tissue_pre)
hiit_cross_id = [
## Healthy
"C16",
"C17",
"C18",
"C21",
"C30",
"C33",
## Tumor
"GU0700",
"GU0715",
"GU0744",
"NPC_SC_1802",
"NPC_SC_1805",
"NPC_SC_1806",
"NPC_SC_1807",
"NPC_SC_1808",
"NPC_SC_1810",
"NPC_SC_1811",
"NPC_SC_1813",
"NPC_SC_1815",
"NPC_SC_1816",
'GC06',
'GC07',
'GC08',
'GC10',
'B230093',
'B230498',
'B230723',
'B231260',
'B357818',
'PT_1',
'PT_2',
'PT_3',
'PT_4',
'PT_5',
'PT_6',
'PT_7',
'PT_8',
## Inflam
"U34",
"U35",
"U4",
"U41",
"U44",
"U45",
"U5",
## irAE
"irAE_40",
"irAE_45",
"irAE_56",
"irAE_62",
"irAE_63",
"irAE_65",
"irAE_7",
"irAE_76",
]
adata_hiit_cross = adata_hiit[adata_hiit.obs['individual_id'].isin(hiit_cross_id)]
clone_circ_cross = clone_circ[(clone_circ['individual_id'].isin(hiit_cross_id))].copy()
clone_tissue_cross = clone_tissue[(clone_tissue['individual_id'].isin(hiit_cross_id))].copy()
clone_hiit_cross = pd.merge(clone_circ_cross, clone_tissue_cross, on='cc_aa_identity')
clone_hiit_cross['total'] = clone_hiit_cross['total_x']+clone_hiit_cross['total_y']
clone_hiit_cross['all'] = 'all'
Counter(adata_hiit_cross.obs['study_name'])
Counter({'Shi_2022': 8263,
'Sun_2022': 8982,
'Liu_2022': 14265,
'Boland_2020': 32990,
'Liu_2021': 39360,
'Borcherding_2021': 4563,
'Kim_2022': 20507})
clone_merge_all_healthy = clone_hiit_cross[clone_hiit_cross['disease_type_x']=='Healthy']
clone_merge_all_inflam = clone_hiit_cross[clone_hiit_cross['disease_type_x']=='Inflammation']
clone_merge_all_irae = clone_hiit_cross[clone_hiit_cross['disease_type_x']=='irAE_inflammation']
clone_merge_all_stumor = clone_hiit_cross[clone_hiit_cross['disease_type_x']=='Solid tumor']
clone_merge_all_inflam = clone_merge_all_inflam[(clone_merge_all_inflam['Circulating']>=1) &
(clone_merge_all_inflam['Tissue']>=1)]
clone_merge_all_irae = clone_merge_all_irae[(clone_merge_all_irae['Circulating']>=1) &
(clone_merge_all_irae['Tissue']>=1)]
clone_merge_all_stumor = clone_merge_all_stumor[(clone_merge_all_stumor['Circulating']>=1) &
(clone_merge_all_stumor['TIL']>=1)]
TIL-circ-sharing¶
adata_hiit_cross_circ = adata_hiit_cross[(adata_hiit_cross.obs['meta_tissue_type_aggr']=='Circulating')]
adata_hiit_cross_tissue = adata_hiit_cross[(adata_hiit_cross.obs['meta_tissue_type_aggr']=='Tissue')]
adata_hiit_cross_til = adata_hiit_cross[(adata_hiit_cross.obs['meta_tissue_type_aggr']=='TIL')]
clone_id_subset = np.unique(clone_merge_all_stumor.index)
adata_share_stumor = adata_hiit_cross_til[
adata_hiit_cross_til.obs['cc_aa_identity'].isin(clone_id_subset)]
adata_share_stumor_reverse = adata_hiit_cross_til[
(~adata_hiit_cross_til.obs.index.isin(adata_share_stumor.obs.index))
]
adata_hiit_cross_til.obs['circulating']=None
adata_hiit_cross_til.obs.loc[adata_share_stumor.obs.index, 'circulating'] = 'Sharing'
adata_hiit_cross_til.obs.loc[adata_hiit_cross_til.obs['circulating'].isna(), 'circulating'] = 'NoSharing'
fig,ax = plt.subplots(figsize=(5,5))
sc.pl.umap(adata_hiit_cross_til, color='circulating', palette=['#37c2ed','#ff8fbd'],
ax=ax)
fig.savefig('figures/NA_sharing.png', dpi=600)
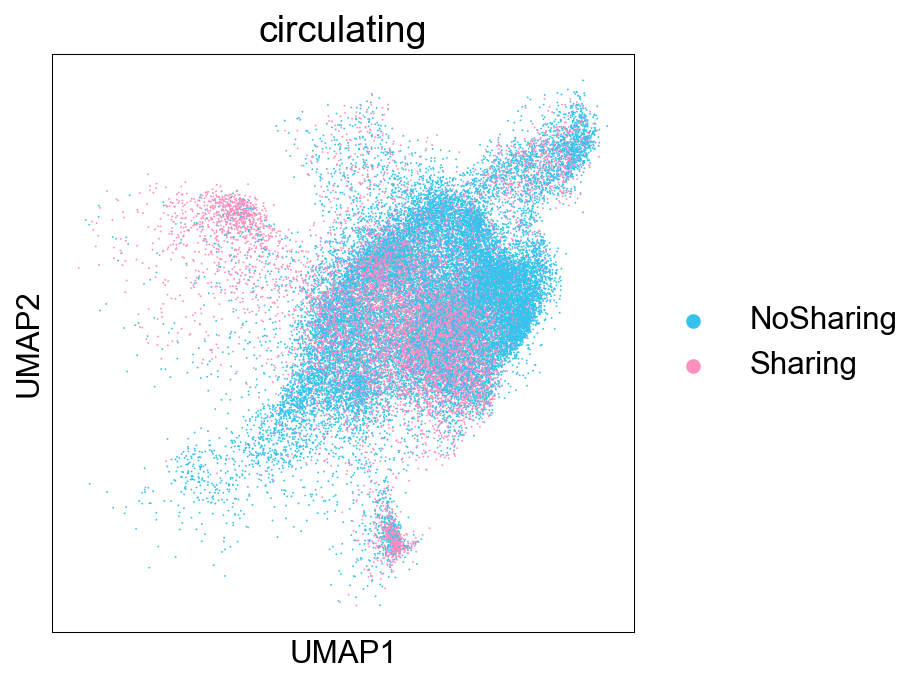
adata_til_cross_allg = adata_gene[adata_gene.obs.index.isin(adata_hiit_cross_til.obs.index)]
adata_til_cross_allg.obs = adata_hiit_cross_til.obs.copy()
adata_til_cross_allg.uns['log1p']['base'] = None
adata_til_cross_allg
AnnData object with n_obs × n_vars = 37429 × 19957
obs: 'sample_name', 'study_name', 'label_1', 'label_2', 'label_3', 'label_4', 'atlas_name', 'leiden', 'class', 'reanno', 'cell_subtype', 'leiden_n_neighbors_30_reoslution_0.6', 'leiden_n_neighbors_30_reoslution_0.8', 'leiden_n_neighbors_30_reoslution_1.0', 'leiden_n_neighbors_30_reoslution_1.2', 'leiden_n_neighbors_50_reoslution_0.6', 'leiden_n_neighbors_50_reoslution_0.8', 'leiden_n_neighbors_50_reoslution_1.0', 'leiden_n_neighbors_50_reoslution_1.2', 'leiden_n_neighbors_15_reoslution_0.6', 'leiden_n_neighbors_15_reoslution_0.8', 'leiden_n_neighbors_15_reoslution_1.0', 'leiden_n_neighbors_15_reoslution_1.2', 'leiden_n_neighbors_40_reoslution_0.6', 'leiden_n_neighbors_40_reoslution_0.8', 'leiden_n_neighbors_40_reoslution_1.0', 'leiden_n_neighbors_40_reoslution_1.2', 'cell_subtype_revision', 'predictions', 'IR_VJ_1_junction_aa', 'IR_VDJ_1_junction_aa', 'IR_VJ_1_v_call', 'IR_VJ_1_j_call', 'IR_VDJ_1_v_call', 'IR_VDJ_1_j_call', 'disease', 'disease_type', 'disease_status', 'tissue_type', 'meta_tissue_type', 'individual_id', 'cell_subset', 'COVID19_status', 'treatment', 'treatment_status', 'donor_sex', 'donor_age', 'A_1', 'A_2', 'B_1', 'B_2', 'C_1', 'C_2', 'meta_cell_subset', 'cell_subtype2', 'meta_cell_subtype', 'tcr', 'cc_aa_identity', 'meta_tissue_type_aggr', 'cell_subtype_3', 'treatment_status_detail', 'cc_aa_identity_size', 'expansion_level', 'expansion_size', 'D50_cc_aa_identity', 'clone_umap', 'circulating'
uns: 'log1p'
obsm: 'X_gex', 'X_umap'
sc.tl.rank_genes_groups(adata_til_cross_allg, groupby='circulating',
groups=['Sharing'], reference='NoSharing')
WARNING: Default of the method has been changed to 't-test' from 't-test_overestim_var'
fig,ax=ScanpyVolcanoPlot(
adata_til_cross_allg, axis=0,
label_fold_change=0.25, label_log_p=2,
label_size=6 ,
label_excludes=list(adata_til_cross_allg.var.index),
label_includes=[
'CCL5',
'CCL4',
'CCL4L2',
'KLRK1',
'KLRD1',
'KLRG1',
'ANXA1',
'CD52',
#control
'CXCL13',
'ENTPD1',
'HAVCR2',
'TOX',
'PDCD1',
],
color2='#ff8fbd',
color1='#2cbef7',
size_by='circulating',
size_group='Sharing',
size_reference='NoSharing'
)
fig.set_size_inches((4,4))
fig.savefig('figures/NNB1_sharing_volcano.pdf')
Fraction of gene CCL5 is 0.9923615531508593
Fraction of gene CCL4 is 0.7654360280076384
Fraction of gene CCL4L2 is 0.5196530872056015
Fraction of gene KLRK1 is 0.37643220878421385
Fraction of gene KLRD1 is 0.3232813494589433
Fraction of gene KLRG1 is 0.26965308720560155
Fraction of gene ANXA1 is 0.7740292807129217
Fraction of gene CD52 is 0.9147835773392744
Fraction of gene CXCL13 is 0.09540101845957989
Fraction of gene ENTPD1 is 0.0519573520050923
Fraction of gene HAVCR2 is 0.09778803309993635
Fraction of gene TOX is 0.160964353914704
Fraction of gene PDCD1 is 0.1558720560152769
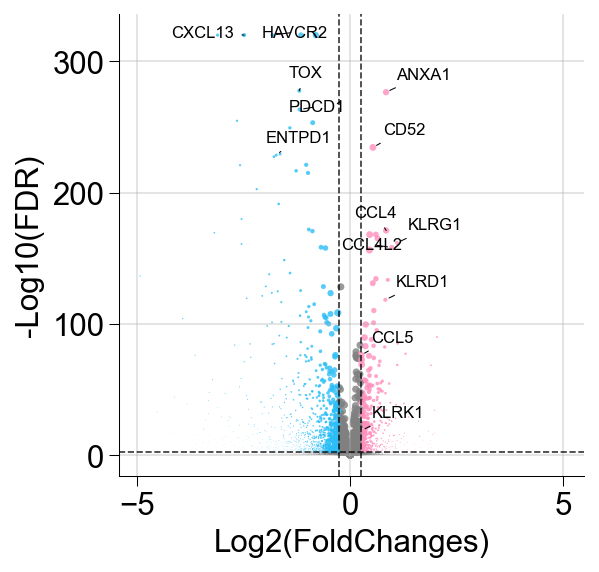
fig,ax=plt.subplots(figsize=(5,5))
sc.pl.umap(adata_hiit_cross_til, color='CXCL13', size=5, cmap='Reds', ax=ax,)
fig.savefig('figures/sharing_CXCL13.png', dpi=600)
fig,ax=plt.subplots(figsize=(5,5))
sc.pl.violin(adata_hiit_cross_til, keys='CXCL13', ax=ax,
groupby='circulating', stripplot=False)
fig.savefig('figures/sharing_CXCL13_violin.pdf')
fig,ax=plt.subplots(figsize=(5,5))
sc.pl.umap(adata_hiit_cross_til, color='ENTPD1', size=5, cmap='Reds', ax=ax,)
fig.savefig('figures/sharing_ENTPD1.png', dpi=600)
fig,ax=plt.subplots(figsize=(5,5))
sc.pl.violin(adata_hiit_cross_til, keys='ENTPD1', ax=ax,
groupby='circulating', stripplot=False)
fig.savefig('figures/sharing_ENTPD1_violin.png')
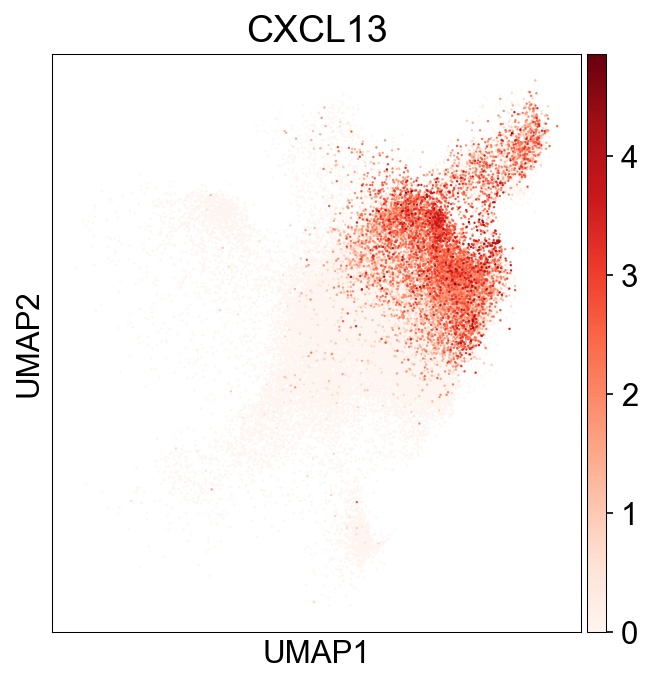
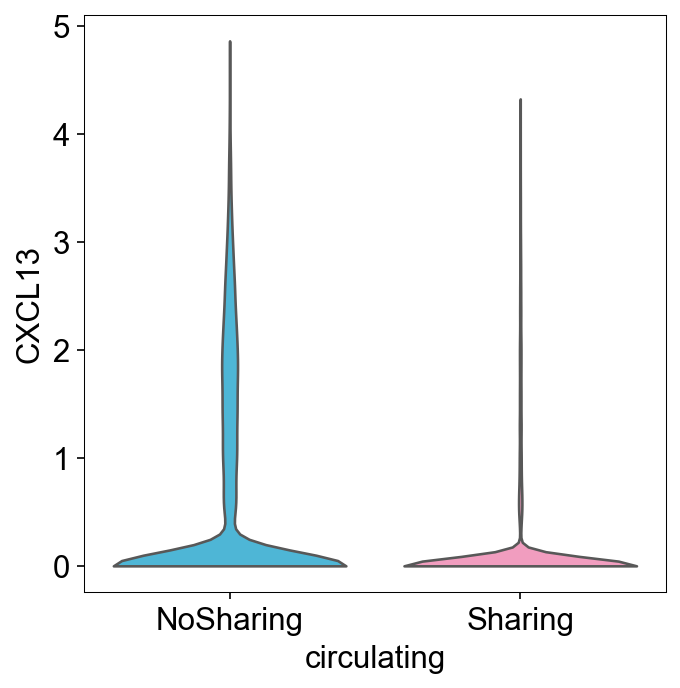
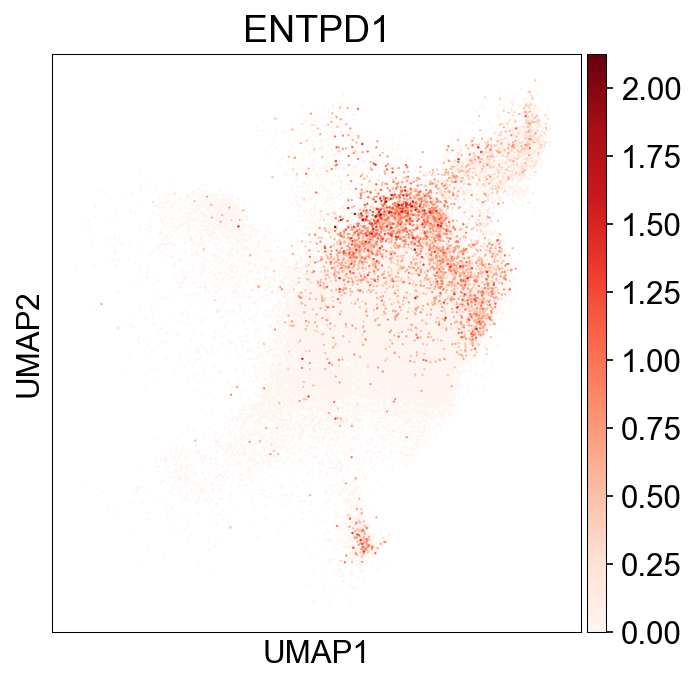
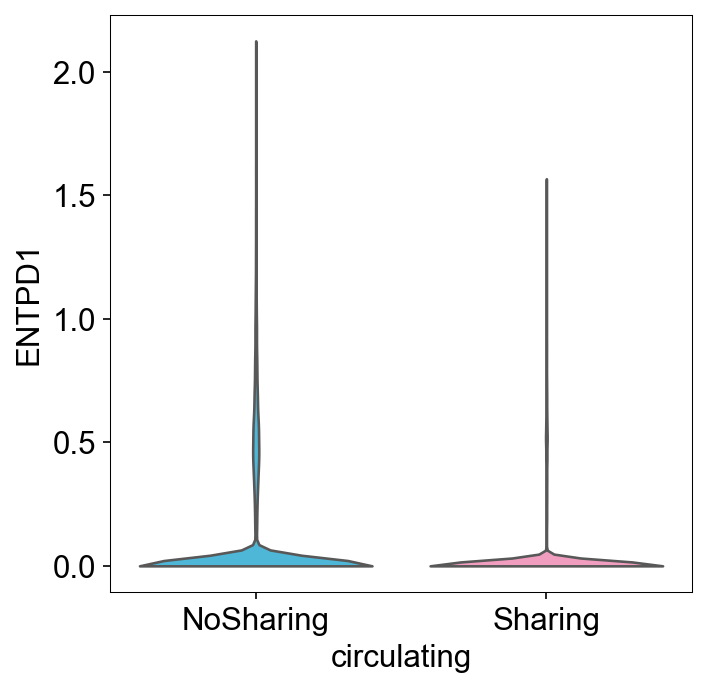
SCENIC analysis¶
Many codes from the scenic analysis part are based on the SCENIC tutorial (https://github.com/aertslab/SCENICprotocol/blob/master/notebooks/SCENIC Protocol - Case study - Cancer data sets.ipynb)
Reference datasets from the cisTarget database are available at https://resources.aertslab.org/cistarget/databases/.
Transcription factors list is available at https://github.com/aertslab/pySCENIC/
import os, glob, re, pickle
from functools import partial
from collections import OrderedDict
import operator as op
from cytoolz import compose
import pandas as pd
import seaborn as sns
import numpy as np
import scanpy as sc
import anndata as ad
import matplotlib as mpl
import matplotlib.pyplot as plt
from pyscenic.export import export2loom, add_scenic_metadata
from pyscenic.utils import load_motifs
from pyscenic.transform import df2regulons
from pyscenic.aucell import aucell
from pyscenic.binarization import binarize
from pyscenic.rss import regulon_specificity_scores
from pyscenic.plotting import plot_binarization, plot_rss
from IPython.display import HTML, display
# Downloaded fromm pySCENIC github repo: https://github.com/aertslab/pySCENIC/tree/master/resources
HUMAN_TFS_FNAME = os.path.join(RESOURCES_FOLDERNAME, 'lambert2018.txt')
# Ranking databases. Downloaded from cisTargetDB: https://resources.aertslab.org/cistarget/
RANKING_DBS_FNAMES = ['./data/hg38__refseq-r80__500bp_up_and_100bp_down_tss.mc9nr.genes_vs_motifs.rankings.feather']
# Motif annotations. Downloaded from cisTargetDB: https://resources.aertslab.org/cistarget/
MOTIF_ANNOTATIONS_FNAME = os.path.join(AUXILLIARIES_FOLDERNAME, './data/motifs-v9-nr.hgnc-m0.001-o0.0.tbl')
def derive_regulons(motifs, db_names=('hg38__refseq-r80__500bp_up_and_100bp_down_tss.mc9nr.genes_vs_motifs.rankings')):
# motifs.columns = motifs.columns.droplevel(0)
def contains(*elems):
def f(context):
return any(elem in context for elem in elems)
return f
# For the creation of regulons we only keep the 10-species databases and the activating modules. We also remove the
# enriched motifs for the modules that were created using the method 'weight>50.0%' (because these modules are not part
# of the default settings of modules_from_adjacencies anymore.
motifs = motifs[
np.fromiter(map(compose(op.not_, contains('weight>50.0%')), motifs.Context), dtype=bool) & \
# np.fromiter(map(contains(*db_names), motifs.Context), dtype=np.bool) & \
np.fromiter(map(contains('activating'), motifs.Context), dtype=bool)
]
# We build regulons only using enriched motifs with a NES of 3.0 or higher; we take only directly annotated TFs or TF annotated
# for an orthologous gene into account; and we only keep regulons with at least 10 genes.
regulons = list(filter(lambda r: len(r) >= 10, df2regulons(motifs[
(motifs['NES'] >= 1.0)
& ((motifs['Annotation'] == 'gene is directly annotated')
| (motifs['Annotation'].str.startswith('gene is orthologous to')
& motifs['Annotation'].str.endswith('which is directly annotated for motif')))
])))
# Rename regulons, i.e. remove suffix.
return list(map(lambda r: r.rename(r.transcription_factor), regulons))
SCENIC for irAE analysis¶
adata_irae_all = sc.read_h5ad('/Volumes/rsch/GEXnotebook/source/adata_irae.h5ad')
adata_subset_irae_all = adata_all_genes[adata_irae_all.obs.index]
EXP_MTX_QC_FNAME = "./data/huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.irae_all.csv"
df_exp = adata_subset_irae_all.to_df()
df_exp.to_csv(EXP_MTX_QC_FNAME)
!pyscenic \
grn ./huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.irae_all.csv \
./data/lambert2018.txt \
-o ./data/pyscenic_adjacencies.irae_all.tsv \
--num_workers 64
!pyscenic ctx ./data/pyscenic_adjacencies.tex_major.tsv \
./data/hg19-500bp-upstream-10species.mc9nr.genes_vs_motifs.rankings.feather \
./data/hg19-tss-centered-10kb-10species.mc9nr.genes_vs_motifs.rankings.feather \
./data/hg19-tss-centered-5kb-10species.mc9nr.genes_vs_motifs.rankings.feather \
--annotations_fname ./data/motifs-v9-nr.hgnc-m0.001-o0.0.tbl \
--expression_mtx_fname ./data/huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.irae_all.csv \
--output ./data/pyscenic_motifs.irae_all.csv \
--num_workers 64
df_motifs = load_motifs('./data/pyscenic_motifs.irae_all.csv')
regulons = derive_regulons(df_motifs)
# Pickle these regulons.
REGULONS_DAT_FNAME = './data/pyscenic_regulons.irae_all.dat'
with open(REGULONS_DAT_FNAME, 'wb') as f:
pickle.dump(regulons, f)
AUCELL_MTX_FNAME = './data/pyscenic_auc.irae_all.csv'
auc_mtx = aucell(df_exp, regulons, num_workers=64)
auc_mtx.to_csv(AUCELL_MTX_FNAME)
add_scenic_metadata(adata_subset_irae_all, auc_mtx, regulons)
df_obs = adata_subset.obs
signature_column_names = list(df_obs.select_dtypes('number').columns)
signature_column_names = list(filter(lambda s: s.startswith('Regulon('), signature_column_names))
df_scores = df_obs.loc[df_obs['cell_subtype_3'].isin(['GZMK+ Tex','ITGAE+ Tex']),
signature_column_names + ['cell_subtype_3']]
df_results = ((df_scores.groupby(by='cell_subtype_3').mean() - df_obs[signature_column_names].mean())/ df_obs[signature_column_names].std()).stack().reset_index().rename(columns={'level_1': 'regulon', 0:'Z'})
df_results['regulon'] = list(map(lambda s: s[8:-1], df_results.regulon))
df_results[(df_results.Z >= 0)].sort_values('Z', ascending=False).head()
df_heatmap = pd.pivot_table(data=df_results[df_results.Z >= 0.5].sort_values('Z', ascending=False),
index='cell_subtype_3', columns='regulon', values='Z')
#df_heatmap.drop(index='Myocyte', inplace=True) # We leave out Myocyte because many TFs are highly enriched (becuase of small number of cells).
cm = sns.clustermap(df_heatmap.fillna(0), fmt=".1f", linewidths=.7,
cbar=False, linecolor='gray', yticklabels=True, xticklabels=True,
cmap="YlGnBu", annot_kws={"size": 12})
cm.fig.set_size_inches(9,1)
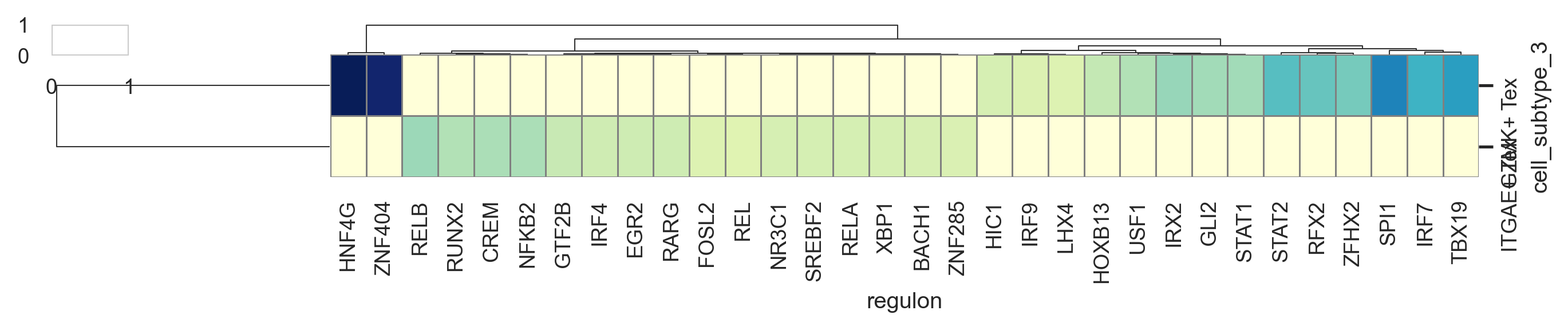
SCENIC for Tex subset analysis¶
adata_tex = sc.read_h5ad("/Users/snow/Desktop/2023-NM-Revision-GEX-HuARdb-Notebooks/data/20231210Tex.retrain_vae.h5ad")
adata_subset_tex = adata_all_genes[adata_tex.obs.index]
EXP_MTX_QC_FNAME = "./data/huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.tex.csv"
df_exp = adata_subset_tex.to_df()
df_exp.to_csv(EXP_MTX_QC_FNAME)
!pyscenic \
grn ./huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.tex.csv \
./data/lambert2018.txt \
-o ./data/pyscenic_adjacencies.tex.tsv \
--num_workers 64
!pyscenic ctx ./data/pyscenic_adjacencies.tex_major.tsv \
./data/hg19-500bp-upstream-10species.mc9nr.genes_vs_motifs.rankings.feather \
./data/hg19-tss-centered-10kb-10species.mc9nr.genes_vs_motifs.rankings.feather \
./data/hg19-tss-centered-5kb-10species.mc9nr.genes_vs_motifs.rankings.feather \
--annotations_fname ./data/motifs-v9-nr.hgnc-m0.001-o0.0.tbl \
--expression_mtx_fname ./data/huARdb_v2_GEX.CD8.all_genes.subset.scenic_qc.tex.csv \
--output ./data/pyscenic_motifs.tex.csv \
--num_workers 64
df_motifs = load_motifs('./data/pyscenic_motifs.tex.csv')
regulons = derive_regulons(df_motifs)
# Pickle these regulons.
REGULONS_DAT_FNAME = './data/pyscenic_regulons.tex.dat'
with open(REGULONS_DAT_FNAME, 'wb') as f:
pickle.dump(regulons, f)
AUCELL_MTX_FNAME = './data/pyscenic_auc.tex.csv'
auc_mtx = aucell(df_exp, regulons, num_workers=64)
auc_mtx.to_csv(AUCELL_MTX_FNAME)
add_scenic_metadata(adata_subset_tex, auc_mtx, regulons)
df_heatmap = pd.pivot_table(data=df_results[df_results.Z >= 0.25].sort_values('Z', ascending=False),
index='cell_subtype_4', columns='regulon', values='Z')
#df_heatmap.drop(index='Myocyte', inplace=True) # We leave out Myocyte because many TFs are highly enriched (becuase of small number of cells).
cm = sns.clustermap(df_heatmap.fillna(0), fmt=".1f", linewidths=.7,
cbar=False, linecolor='gray', xticklabels=True,
cmap="YlGnBu", annot_kws={"size": 12})
cm.fig.set_size_inches(28,2)
# fig.yticks(rotation=90)
cm.fig.savefig("./figures/PyScenic_TEX_enrichment.pdf")
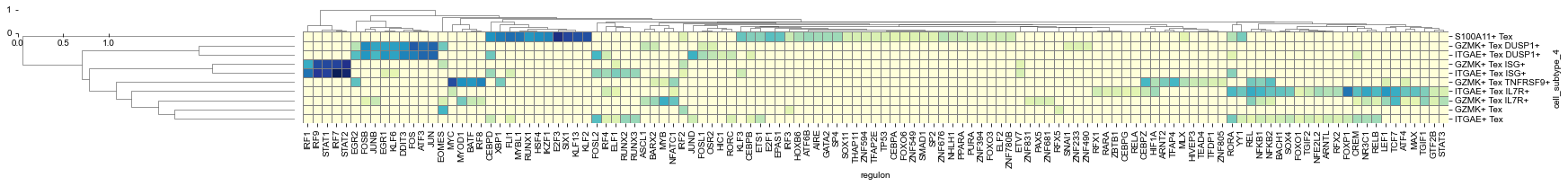
selecting TF-genes for regulatory network¶
node_edge_dict = {'nodes':[],'links':[]}
snode = set()
df_heatmap = df_results[np.array(df_results.Z >= 0)].sort_values('Z', ascending=False).head(32)
s = set(df_heatmap['regulon'])
for i in list(filter(lambda x: x.name in s, regulons)):
tf = i.name
if all(map(lambda x: x < 0, i.gene2weight.values())):
continue
node_edge_dict['nodes'].append(dict(
id=tf,
group='TF'
))
snode.add(tf)
for j,w in i.gene2weight.items():
if w >= 5:
if j not in snode:
node_edge_dict['nodes'].append(dict(
id=j,
group='G'
))
snode.add(j)
node_edge_dict['links'].append(dict(
source=tf,
target=j,
value=w
))
### node_edge_dict is used for visualization the network